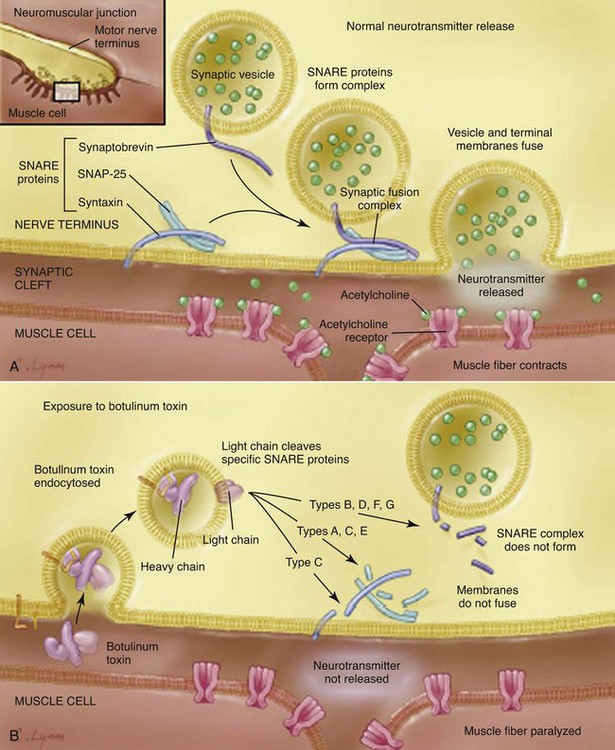
Botulism is a neuroparalytic illness resulting from intoxication with a neurotoxin produced most commonly by Clostridium botulinum. C. botulinum is a group of culturally distinct organisms that are alike only in that they are clostridia that produce toxin with the same pharmacologic properties, even though the toxins themselves are antigenically distinct.13,40,40 C. botulinum organisms are gram-positive, straight to slightly curved, motile, spore-forming, saprophytic, anaerobic rods that are distributed in soil worldwide.13,14 They are 0.5 to 2.0 µm in width and 1.6 to 22 µm in length, with oval subterminal spores.13 The spores are resistant to heat, light, drying, chemicals, and radiation. Anaerobic conditions and the appropriate nutrient environment (high quantities of organic material) are necessary for spore germination and cell division. The organism grows best under anaerobic conditions and with warmth (15° C to 45° C), although some strains can grow at temperatures as low as 6° C. The organism produces toxin under anaerobic, low-acid (pH over 4.6), and low-solute conditions.59 Seven antigenically distinct types of botulinal neurotoxin (BoNT) have been identified: A, B, C, D, E, F, and G.13,29 All of these types have similar structure and the same neurotoxic effect. The nucleotide sequences for all types have been determined,2,59 but the three-dimensional structure of only type A is known.40 Types A, B, E, and F are associated with human disease.10,30 Type D is most prevalent in herbivores. Type C is more prevalent in birds and carnivores.13,59 All canine and feline cases to date have been caused by type C toxin, with the exception of two canine cases of type D reported from Senegal.23,24 Type C botulism has been reported in lions (Panthera leo), but jaguars (Panthera onca) and coatis (Nasua nasua), eating the same food as the lions did, were not affected.5,18 Usually a single organism expresses a single toxin type.14 Types C and D toxins are produced by a C. botulinum group that is partially proteolytic and does not ferment glucose.14 Although mildly alkaline pH stimulates growth of most types of C. botulinum, pH 5.7 may be optimal for type C–producing strains. Botulinal toxins are the most potent, naturally occurring, acutely toxic substances known with an LD50 of 1 to 5 ng/kg.48,56 In people, botulinal toxin is 10,000 times more toxic than cyanide8 and 15,000 times more potent than sarin gas.59 Botulinal toxin is released from vegetative cells only by lysis of the cell or spore. The toxin is not secreted from the cell or spore.14 The amount of toxin in spores is only approximately 1% of that found in vegetative cells, but the intrasporal toxin is resistant to heat denaturation. Type C toxin is stable at a pH range from 2.7 to 10.2.34 Vegetative cells produced by germinating spores begin to produce toxin within several days after germination. Botulism is usually caused by ingestion of the preformed toxin. In people, this is usually due to improperly canned or preserved food. Other forms of botulism in humans include infant intestinal botulism (the most common form of the disease in humans), adult intestinal botulism, wound botulism, inadvertent injection botulism, and inhalational botulism. Infant botulism is caused by in vivo production of toxin in the intestine after ingestion and then germination of spores and intestinal colonization with neurotoxigenic clostridia, not necessarily C. botulinum.10,13 Normal human adults are resistant to intestinal colonization with C. botulinum, largely because of intestinal microflora. Colonization can be induced in adult animals or people only if they are germ-free or are being treated with antibacterials.5 Persistent intestinal colonization in adults with C. botulinum rarely causes clinical illness.5 Intestinal colonization was established experimentally in 8- to 11-day-old puppies, but no intoxication occurred.5 Intestinal colonization has been found in a natural case of botulism in a 6-month-old dog.5 However, the dog recovered without antibacterial therapy, even though intestinal colonization continued for several months. Wound botulism, in which the organisms infect a wound and produce toxin, is a rare form of botulism in people, usually associated with illicit, injectable drug use.13 Injection-related botulism is a potential adverse event following injection of pharmaceutical botulinal toxin (e.g., Botox or Myobloc).38 Inhalational botulism has been described in three laboratory workers after performing a necropsy on an animal that died of botulism.38 Although botulinal toxin has never been used successfully for warfare or bioterrorism, concern exists in regard to its potential as a bioweapon.38 The source of botulinal toxin is rarely found in reported canine cases. Type C toxin has been isolated from fly larvae (maggots) and from carrion, and most canine cases are thought to be associated with ingestion of carrion.26,30,30 Other canine cases were associated with wetlands during warm weather, the areas associated with avian botulism epizootics.52 An outbreak in cats was associated with ingestion of a pelican carcass.25 All the different serotypes of BoNT are a single polypeptide metalloproteinase of 150 kDa.2,56 All the BoNT are composed of a heavy (H) chain of 100 kDa and a light (L) chain of 50 kDa. The H chain consists of an amino-terminal domain of 50 kDa and a carboxy-terminal end of 50 kDa.13 The H and L chain are bridged by a single interchain disulfide bond.2,35 The interchain disulfide bond is essential for toxicity.55 BoNT is inactive when initially released. Activation is achieved by a specific proteolytic cleavage of a single peptide bond within a surface exposed loop.2,55 Bacterial or host proteases can accomplish this cleavage. After cleavage, the toxin remains as an associated H and L chain via the disulfide bridge and via noncovalent protein-protein interactions.50 Botulinal toxins are produced as progenitor toxins, the toxin bound noncovalently with nontoxic proteins. The progenitor toxins are released on bacterial autolysis.56 The progenitor toxin is very stable at low pH, protecting the neurotoxin from proteolytic attack in the gastric environment.56 This stability allows the complex to reach the small intestine, where the alkaline pH is conducive to dissociation of the complex, releasing the neurotoxin,14,40 which explains why the progenitor toxin is more toxic than neurotoxin alone when administered orally.35 The number and size of the associated nontoxic proteins differ between toxin types. Type C toxin may be associated with up to five additional proteins, resulting in a released complex of molecular size of 500 or 900 kDa.14 Once ingested, types C and D toxins are absorbed primarily from upper small bowel into the lymphatic system via endocytosis similar to nutrient proteins. Toxin can continue to be absorbed from the lower small bowel and colon, but this process proceeds more slowly. From the lymphatic system, the toxin enters the bloodstream. One reason for differences in susceptibility to toxins is the ability of hemagglutinin (HA) proteins in the toxin complex to disrupt the paracellular barrier of the intestinal epithelium.41 Types A and B HA proteins disrupt the human intestinal epithelial cell barrier, whereas type C HA proteins are unable to disrupt this barrier, but can disrupt and cause cytotoxicity of canine epithelial cell lines.41 The absorbed toxin is in its disulfide-bonded di-chain form.14 Botulinal toxin then enters tissue fluids, reaching the peripheral cholinergic nerve terminals.56 The high molecular weight of botulinal toxin prevents its crossing the blood-brain barrier so that all neurologic symptoms of botulism are related to the peripheral nervous system.38 The basis of the production of lower motor neuron (LMN) paralysis in botulism is that the toxin prevents the presynaptic release of acetylcholine at the neuromuscular junction. Both the spontaneous release of acetylcholine and its release caused by a nerve action potential are inhibited.40 Signs of autonomic dysfunction (parasympathetic and sympathetic) coexist with skeletal neuromuscular dysfunction, but are milder in severity.38,40 Neural intoxication occurs in four steps: rapid and specific binding to cell surface receptors by the carboxy terminus of the H chain, internalization of the toxin into endosomal-like compartments, membrane translocation of the L chain into the cytosol facilitated by the amino terminus of the H chain, and enzymatic cleavage of target proteins by the L chain (Fig. 40-1). The carboxy-terminal end of the H chain is responsible for binding to the presynaptic membrane (see Fig. 40-1).35,49 This end of the H chain is composed of two subdomains, an N terminus and a C terminus.14,37 The C-terminal end is the most critical to binding and is the most variable between toxin types, perhaps explaining part of the difference in species susceptibility to different toxins.37,55 Binding occurs very quickly and is irreversible, unaffected by temperature, and independent of neural activity. The cell membrane receptors for the toxin must have very high affinity for it because only minute quantities (fewer than 10−12 mol) of botulinal toxin are sufficient to cause death.30,35 The receptor sites on the neuron are not well understood, but it is postulated that binding requires both polysialoganglioside and other protein components.13,14,14 Variations in receptor affinity for different types of toxin also help explain the different sensitivities of animal species to the different botulinal toxins.30,35,35 During this stage, the toxin is susceptible to inactivation by antitoxin. After binding, the toxin passes through the cell membrane by receptor-mediated endocytosis.10 Once inside the cell, the toxin is more resistant to inactivation by antitoxin. However, some experiments show that some types of antibody can enter cholinergic neurons and inactivate internalized neurotoxin.2 The toxin is internalized inside a vesicle endowed with an ATPase proton pump.48,56 The amino-terminal end of the H chain governs toxin translocation (see Fig. 40-1). This process is temperature and energy dependent.49 The translocation is pH dependent and requires a low-pH step. Low pH triggers a change in the toxin to a state of greater hydrophobicity, leading to ability to penetrate the membrane lipid bilayer.14 Membrane translocation is triggered by acidification of the lumen of the vesicle, which causes the L-chain to be released from the vesicle into the cytosol.56 The free L-chain, a zinc-containing metalloproteinase enzyme, is the most metabolically active protein of the toxin molecule.33a The blockage of acetylcholine release is caused by zinc-dependent cleavage of one or two of the three core protein components of the neuroexocytosis apparatus (the SNARE proteins).14,40,40 The SNARE proteins are essential to the docking and fusion of synaptic vesicles with the presynaptic membrane, events that lead to the release of acetylcholine into the neuromuscular junction (see Fig. 40-1).13,14 A remarkable finding is that proteolysis of a small amount of the total SNARE present is sufficient to block acetylcholine release.55 Different types of botulinal toxin cleave different bonds within this membrane protein system. Differences in the targets for the toxins between species are also thought to account for species insensitivity to certain toxin types. Type C toxin targets syntaxin and SNAP-25 proteins, with syntaxin cleavage being most significant.40,55 Syntaxin cleavage by type C toxin prevents G-protein regulation of calcium channels associated with presynaptic neurotransmitter release sites. Normally, calcium influx through these ion channels stimulates fusion of the vesicles and release of neurotransmitter into the synapse. Thus, with type C toxin, the formation of synaptic vesicles, their number, and their distribution along the presynaptic membrane are all normal, but neurotransmitter cannot be released because the vesicles cannot fuse with the presynaptic membrane.2 The lifetime of intracellular toxin can be quite long, and until the last molecule of the L chain is degraded, toxin-mediated SNARE proteolysis will occur.55 The cleaved SNAP-25 proteins also inhibit neurotransmitter release and are very stable.48,56 The more active the neuron, the more rapid its inhibition.48,56 The duration of the inhibitory effect of BoNTs varies with toxin type, dose, type of nerve terminal, and animal species.56 The inhibitory effect of BoNT is not limited to Ach, affecting the release of other neurotransmitters and neuropeptides.56 In all species, botulism is characterized by generalized LMN dysfunction, leading to weakness and flaccid paralysis.14 Autonomic dysfunction also occurs. In the most severe cases, failure of respiratory muscles leads to death. Botulism should be considered in dogs and cats with diffuse LMN disease. The clinical signs in type C canine botulism have been the same whether experimentally or naturally induced.5,8,52,61 The severity of signs varies with the amount of toxin ingested and individual susceptibility. The first sign is a progressive, symmetric, ascending weakness from the rear to the forelimbs that can result in quadriplegia (Fig. 40-2). Tail wag is maintained. A complete neurologic examination will show hyporeflexia and hypotonia, indicating generalized LMN dysfunction. Cranial nerves are often affected: mydriasis with sluggish pupillary responses, decreased jaw tone, decreased gag reflex with excess salivation, diminished palpebral reflexes, and weak vocalization have been found in affected dogs. Pain perception and alert mental attitude are maintained (Fig. 40-3). In severely affected dogs, decreased abdominal muscle tone and primarily diaphragmatic respiration may occur.
Botulism
Etiology
Pathogenesis
Clinical Findings
Dog
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Botulism