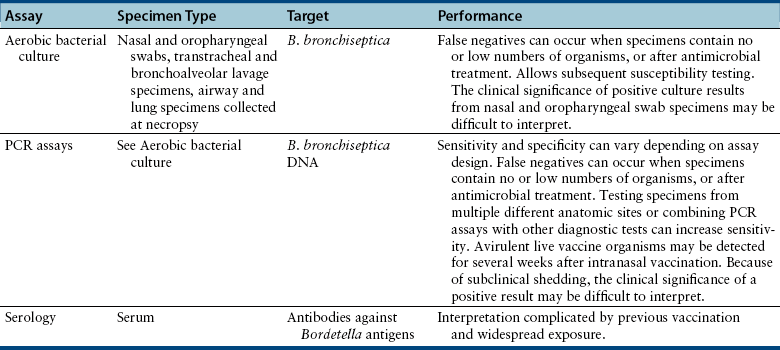
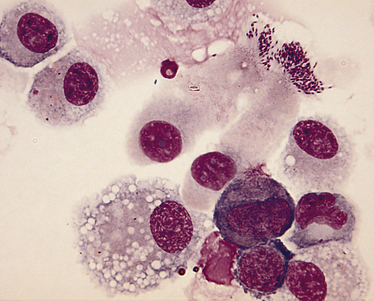
Chapter 38 Bordetella spp. are small, pleomorphic, gram-negative coccobacilli. They have fimbriae (pili) and can be motile by means of flagella. There are at least nine different bacterial species in the genus Bordetella (Table 38-1).3 The only species known to cause disease in dogs and cats is Bordetella bronchiseptica, but B. bronchiseptica is closely related to B. pertussis and B. parapertussis, which cause pertussis (whooping cough) in humans. In fact, there is evidence that B. pertussis evolved from a distinct, human-associated lineage of B. bronchiseptica,4 and it has been recommended that B. bronchiseptica, B. pertussis, and B. parapertussis be reclassified as subspecies of the “B. bronchiseptica cluster.”5 B. pertussis can colonize puppies in the absence of clinical signs of respiratory disease.6 TABLE 38-1 Species That Belong to the Genus Bordetella Worldwide, B. bronchiseptica is an important cause of respiratory disease in dogs and cats; it also infects and causes respiratory illness in many different animal species including wild carnivores, pigs, rabbits, and occasionally horses, other herbivores, rodents, turkeys, and humans. There are a large number of different strains of B. bronchiseptica that vary in virulence and host specificity. Nevertheless, the results of molecular typing efforts have shown that strains that infect dogs can be passed to cats and vice versa.7–9 The whole genome of B. bronchiseptica has been sequenced.10 Its genome is larger than that of its close relatives, and it possesses several plasmids, some of which mediate antimicrobial drug resistance.11 Like viral respiratory infections, bordetellosis is especially prevalent in dogs and cats in shelter, pet stores, boarding facilities, and other situations where large numbers of potentially stressed animals may have been in close contact with one another. Infections with B. bronchiseptica frequently occur in concert with respiratory viral and/or Mycoplasma spp. infections (see Chapters 17, 23, and 40). Unlike B. pertussis and B. parapertussis, B. bronchiseptica can persist in the environment for at least 10 days and can grow in natural water sources,12 but is susceptible to most disinfectants provided they are used correctly. The prevalence of B. bronchiseptica infections in group-housed animals, including shelters, varies from group to group, even in the same geographic region. B. bronchiseptica can be isolated from apparently healthy cats and cats with respiratory disease, but is more likely to be isolated from cats with respiratory disease. In one European study that included 1748 cats from private multicat households, shelters, and breeding catteries, the more cats in the group, the greater the chance that B. bronchiseptica would be detected. In rescue shelters, increased seroprevalence has been associated with poor hygiene.13 In a study of 742 cats in the United Kingdom, B. bronchiseptica was isolated from 0% of pet cats, 19.5% of cats from rescue shelters, and 13.5% of cats from research colonies.14 B. bronchiseptica was cultured from 3.1% of 614 cats from four shelters in Louisiana,15 5.1% of nasal swabs from 59 cats with acute respiratory disease in Colorado,16 and none of 22 cats with respiratory disease in a shelter in California.17 However, the prevalence of infection was nearly 50% in nasal swabs from 40 cats with rhinitis from a shelter in Colorado.18 Similarly, a high prevalence (26%) of infection was detected with a PCR assay in 100 cats with upper respiratory disease from central Italy.19 Only a few studies have examined the epidemiology of B. bronchiseptica infection in dogs. In a case review from the eastern United States, B. bronchiseptica was isolated from 32 (49%) of 65 pet dogs under 1 year of age with radiographic and microbiologic evidence of bacterial bronchopneumonia. Most of these dogs had originated from breeders (40%) or pet stores (41%), and only 8% were from shelters. Dogs infected with B. bronchiseptica were younger (7 to 35 weeks of age), had been owned for a shorter period of time (median, 18 days), were more likely to have originated from a pet store and were less likely to have originated from a breeder than dogs with pneumonia caused by other organisms. B. bronchiseptica was isolated from dogs with respiratory disease as well as from healthy dogs in a rehoming kennel in the United Kingdom.20 Transmission of B. bronchiseptica occurs primarily by the airborne route, but contact with contaminated fomites and water sources may also be important. The organism is highly contagious. Much of what is known about B. bronchiseptica pathogenesis is based on studies of infections in host species other than the dog or cat. B. bronchiseptica is inhaled, adheres to respiratory cilia, evades the immune system, and secretes toxins that damage the respiratory epithelium(Figure 38-1). Adhesion of virulent strains of B. bronchiseptica to respiratory cilia occurs by means of fimbrial adhesins (FIM), a filamentous hemagglutinin (FHA), pertactin, and cell wall lipopolysaccharide. Bordetella colonization factor A (BcfA) is another outer membrane protein that appears to be important for tracheal colonization by B. bronchiseptica.21 B. bronchiseptica possesses genes that encode for pertussis toxin, an important adhesin in B. pertussis. These genes are not expressed in vitro, but there is some evidence for pertussis toxin expression in vivo.22 Evasion of host defenses is mediated by the organism’s outer capsule (which is absent in B. pertussis and B. parapertussis), adenylate cyclase toxin, and pertactin. The capsule protects it from phagocytosis and destruction by complement. Adenylate cyclase toxin catalyzes the conversion of ATP to cAMP, which inhibits the migration and activation of phagocytes and T-lymphocytes. Pertactin allows the organism to resist neutrophil-mediated clearance.23 A type VI secretion system also appears to play a key role in persistence of B. bronchiseptica.24 Other virulence determinants present in the outer membrane include lipopolysaccharide and the Brk protein (Bordetella resistance to killing), which allow the organism to resist complement-mediated destruction. At least two other important exotoxins damage respiratory epithelial cells and cells of the immune system, and incite cytokine production: tracheal cytotoxin (TCT) and dermonecrotic toxin (DNT). B. bronchiseptica also possesses a type III secretion system that allows it to inject as-yet-undefined effector proteins into cellular targets.5 FIGURE 38-1 Bordetella bronchiseptica infection. B. bronchiseptica is inhaled, adheres to respiratory cilia by means of fimbrial adhesins, evades the immune system and secretes a variety of toxins that damage the respiratory epithelium. The inflammation and altered cell function that occur as a result of B. bronchiseptica infection lead to increased fluid and mucus secretion, and impairment in host innate immune defenses predisposes to opportunistic viral and bacterial infections. Clinical signs vary in severity and may reflect factors such as the bacterial strain involved, host immunity, and the presence of co-infections. The incubation period ranges from 2 to 10 days. Rhinitis and tracheobronchitis may be associated with serous to mucopurulent nasal discharge, sneezing, stertor, and, especially in dogs, a persistent, often paroxysmal harsh cough. In some dogs, development of bronchopneumonia leads to fever, productive cough, lethargy, and decreased appetite. Bronchopneumonia may be due to B. bronchiseptica itself or it may result from co-infection with other respiratory pathogens, such as canine distemper virus (CDV). In cats, infection is significantly associated with sneezing, and cough is uncommon.14 Cats with pneumonia may develop tachypnea, cyanosis, and death. Shedding of B. bronchiseptica by both dogs and cats can continue intermittently for at least a month and sometimes several months after infection. Evasion of the immune system through survival inside phagocytes may explain the persistent infection that occurs. In contrast, B. pertussis is rapidly destroyed by macrophages.25 On physical examination, dogs with uncomplicated bordetellosis are bright, alert, and active with a paroxysmal cough that can occur throughout the examination. The cough is often easily elicited on tracheal or laryngeal palpation. Conjunctivitis and serous to mucopurulent ocular and nasal discharge may be present in affected dogs and cats (Figure 38-2). Dogs and cats with complicated Bordetella bronchopneumonia may be pyrexic, lethargic, and tachypneic with increased respiratory effort. There may also be increased lung sounds and/or referred upper airway noises on thoracic auscultation, and mucopurulent nasal discharge. There are no specific laboratory abnormalities in uncomplicated bordetellosis. Usually the CBC is normal. Dogs with bronchopneumonia can also have a normal CBC,26 or a mild to moderate neutrophilia, bandemia, and toxic neutrophils may be present. Cytologic examination of transtracheal lavage (TTL) or bronchoalveolar lavage (BAL) specimens may reveal a suppurative or mixed exudate, and sometimes intracellular and extracellular coccobacilli are visible (Figure 38-3). FIGURE 38-3 Bronchoalveolar lavage cytology from a dog infected with Bordetella bronchiseptica. Numerous coccobacilli are adhered to the cilia of columnar epithelial cells. Wright stain, 1000× oil magnification. (Courtesy of Michael Scott, Michigan State University. In Raskin RE, Meyer D, eds. Canine and Feline Cytology: A Color Atlas and Interpretative Guide, 2 ed, St. Louis, MO: Saunders; 2010.) The main assays used for diagnosis of bordetellosis in dogs and cats are aerobic bacterial culture and PCR assays, which can be performed on nasal or oropharyngeal swabs, TTL or BAL specimens, or respiratory tract tissue collected at necropsy (Table 38-2). In some studies, B. bronchiseptica has been detected in nasal but not oropharyngeal swabs, but in other studies, the use of oropharyngeal swabs was more sensitive. Collection of swab specimens from the nasal cavity or posterior nasopharynx is preferred for diagnosis of pertussis in humans because these regions have ciliated epithelial cells, for which B. pertussis has affinity.27 False-negative results occur when organism numbers in secretions are low, and depending on the sensitivity of the PCR assay used and the viability of the bacteria in the specimen, culture may be positive when PCR is negative and vice versa. Although the detection of B. bronchiseptica in TTL or BAL specimens is diagnostic for bordetellosis, it may be more difficult to interpret the significance of a positive culture or PCR assay result from nasal or oropharyngeal swabs, especially among group-housed dogs and cats when the background prevalence of infection is high. Co-infections with viral respiratory pathogens should always be considered when B. bronchiseptica is detected, as well as the presence of other comorbidities that suppress the innate or adaptive immune system that could predispose to bordetellosis (e.g., ciliary dyskinesia or brachycephalic obstructive syndrome in dogs, retroviral infections in cats).
Bordetellosis
Etiologic Agent and Epidemiology
Species
Host(s)
Disease
Bordetella bronchiseptica
Dogs, cats, pigs, horses, rabbits, rodents, humans
Rhinitis, tracheobronchitis, bronchopneumonia
Bordetella pertussis
Humans
Whooping cough
Bordetella parapertussis
Humans, sheep
Whooping cough (humans); ovine-adapted strains cause respiratory disease in sheep
Bordetella holmesii
Humans
Upper respiratory tract disease, bacteremia
Bordetella trematum
Humans
Otitis media and wound infections
Bordetella avium
Poultry (especially turkey)
Rhinotracheitis
Bordetella hinzii
Birds, rarely humans
Normal flora of birds, bacteremia, cholangitis in humans
Bordetella petrii
Environment, rarely humans
None, osteomyelitis
Bordetella ansorpii
Humans
Bacteremia, skin infection
Clinical Features
Physical Examination Findings
Diagnosis
Microbiologic Tests
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Bordetellosis
Only gold members can continue reading. Log In or Register to continue