CHAPTER 15 Bone Marrow
Bone marrow is the major hematopoietic organ of the body and is responsible for production of erythrocytes, platelets, granulocytes, monocytes, and a small number of lymphocytes. Most blood lymphocytes, however, are derived from the secondary or peripheral lymphoid tissues, including tonsil, lymph nodes, gut-associated lymphoid tissue, bronchial-associated lymphoid tissue, and spleen.
Almost all bones of the body are involved in hematopoiesis at birth. In the neonate, isolated hematopoietic activity also may be present within the spleen and liver. As adulthood approaches, hematopoietic activity is generally restricted to the proximal long bones and axial skeleton (vertebrae, sternebrae, pelvis, ribs).1 Hematopoietic activity may resume within the diaphyses of long bones, spleen, and liver in response to a profound increase in blood cell demand, such as in equine infectious anemia, or to unregulated blood cell production, such as in primary erythrocytosis (polycythemia vera) or leukemia.2,3
Bone Marrow Aspiration
Bone marrow aspiration biopsy is a valuable adjunct in evaluating diseases of hematopoietic tissues. Primary indications for bone marrow examination include anemia, persistent leukopenia, leukocyte cytoplasmic and nuclear maturation abnormalities, persistent thrombocytopenia, unexplained pancytopenia, suspected hematologic neoplasia, and suspicion of bone marrow necrosis or infiltrative disease, including stromal proliferation, infectious agents, or metastatic neoplasia.1,4
Contraindications
Bone marrow aspiration biopsy is a relatively innocuous procedure in horses. Excessive hemorrhage is theoretically possible after bone marrow aspiration, but this is extremely rare.4 If severe thrombocytopenia (<20,000 platelets/μl), clotting factor deficiencies, or disseminated intravascular coagulation is present, any hemorrhage usually can be corrected by applying direct pressure to the aspiration site for 4 to 5 minutes.1 When aspirating marrow from sternebrae, the needle should be placed carefully to prevent cardiac puncture. One horse died after sternal bone marrow aspiration because of left ventricular laceration and subsequent cardiac tamponade. Monoclonal gammopathy and defective hemostasis in this horse probably contributed to development of cardiac tamponade.5 Iatrogenic infection is theoretically possible but is unlikely when the aspiration site is properly prepared and aseptic technique is followed.4
Specimen Collection
A small stab incision is made in the skin with a #11 scalpel blade. The needle is inserted until the periosteum is penetrated and marked resistance encountered. The cortical bone is penetrated by applying steady pressure while rotating the spinal needle. The extent of bone penetration and effort required to obtain bone marrow depend on the site of aspiration and the animal’s age. Generally, 1 to 2 cm of penetration of the tuber coxae are sufficient in the adult, and similar sites in the foal require less penetration. Cortical bone is more resistant to needle penetration in adults, and considerably less effort is required to penetrate cortical bone in foals.
Specimen Preparation and Staining
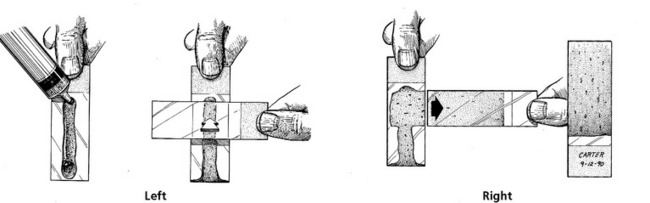
A few drops of marrow suspension are expelled onto a slide that is held vertically (Fig. 15-1). Marrow particles adhere, whereas blood flows down the slide. Squash preparations are made by holding a second slide at a right angle to the first slide and pressing gently while pulling the second slide laterally (Fig. 15-1). Alternatively, the marrow may be expelled into a Petri dish, where particles may be selected with a Pasteur pipette. Particles are placed on a slide, and squash preparations are made as described earlier. For marrow core biopsies, the core can be rolled gently between two slides to produce cytologic specimens before tissue fixation. For necropsy specimens, a small portion of marrow is used to prepare cytologic touch imprints or squash preparations.
Once air-dried, bone marrow specimens may be stained by the classic Wright’s stain technique or rapid modification of the Wright’s stain procedure using Diff-Quik stain. With the classic Wright’s stain, the staining and buffering times should be extended to 5 minutes each because of increased cellularity of the preparations. With the Diff-Quik procedure, bone marrow smears should be dipped in each solution a total of 10 to 20 times to account for increased cellularity. Under low microscopic magnification (10× objective), red, blue, and purple colors should be apparent. Lack of purple color, especially in cell nuclei, indicates insufficient staining. The preparation can be salvaged by repeating the staining procedure on the original smear until the desired tinctorial properties are achieved. (See Chapter 14 for more information on staining procedures and problems.)
Bone Marrow Evaluation
Adequate evaluation of bone marrow aspirates requires familiarity with normal blood cell development and knowledge of the patient’s current complete blood count (CBC) data.6,7 Familiarity with normal blood cell development is necessary to discern abnormalities in blood cell maturation and morphology rapidly, as well as detect unusual cell populations in a bone marrow aspirate. Knowledge of current blood cell data is mandatory to interpret bone marrow response in various disease states. For example, an impression of an increased myeloid/erythroid (M/E) ratio in the presence of nonregenerative anemia and a normal leukogram suggests erythroid hypoplasia, whereas the impression of an increased M/E ratio in the presence of neutrophilia and a normal packed cell volume suggests granulocytic hyperplasia.
Bone marrow evaluation can be accomplished by subjective assessment of the marrow aspirate in most cases. In a clinical setting, calculation of a precise M/E ratio based on a 500-cell differential count is laborious and often of limited value. Therefore, cursory interpretation of bone marrow specimens should be directed toward an impression of the changes consistent with available clinical findings and CBC data. However, bone marrow reference intervals for horses are included for practitioners who want to perform 500-cell differential counts and calculate M/E ratios (Table 15-1).
TABLE 15-1 Bone Marrow Reference Intervals for Horses
| Cell Type | Range (%) | Mean (%) |
|---|---|---|
| Myeloid (Granulocytic) Series | ||
| Myeloblasts | 0–5 | 1.0 |
| Promyelocytes (progranulocytes) | 0.5–3.5 | 1.7 |
| Neutrophils | ||
| Myelocytes | 1.0–7.5 | 3.2 |
| Metamyelocytes | 1.5–15.0 | 5.6 |
| Bands | 6.0–6.5 | 15.7 |
| Segmenters | 3.0–16.5 | 8.4 |
| Eosinophils (total) | 0.0–5.0 | 1.8 |
| Basophils (total) | 0.0–1.0 | 0.3 |
| Total myeloid cells | 26.5–45.0 | 35.7 |
| Erythroid Series | ||
| Rubriblasts, prorubricytes | 0.0–2.0 | 0.7 |
| Rubricytes, metarubricytes | 29.5–89.5 | 55.0 |
| Total erythroid cells | 47.0–69.0 | 58.0 |
| M/E Ratio | 0.48–0.91 | 0.71 |
| Other Cells | ||
| Lymphocytes | 1.5–8.5 | 3.5 |
| Plasma cells | 0.0–2.0 | 0.6 |
| Monocytes, macrophages | 0.0–1.0 | 0.2 |
| Mitotic figures | 0.0–3.5 | 0.8 |
Marrow sample evaluation involves subjective assessment of particle cellularity; megakaryocyte numbers and maturity; and erythroid and myeloid development, maturation, and morphology. In addition, the presence of lymphoid cells, macrophages, stromal cells, and unusual cell populations should be addressed. Examination should proceed in a systematic manner so important changes are not overlooked. Generally, particle cellularity, megakaryocyte numbers, and megakaryocyte maturation are evaluated at lower magnification using the 4× to 20× microscope objectives. Erythroid and myeloid maturation and other critical examinations of cell morphology should be done using the 45× to 50× and 100× microscope objectives.
Particle Cellularity
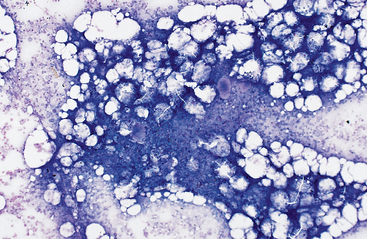
Precise evaluation of bone marrow cellularity requires histologic examination of bone marrow core biopsies taken from a site of active hematopoiesis or disease. Bone marrow core biopsy is not performed routinely in many private practices, so marrow cellularity is assessed cytologically using stained marrow smears and the 10× microscope objective. Bone marrow particles in cytologic preparations consist of fat (appearing as clear spaces or balloon-like adipocytes) and clusters of hematopoietic cells, in which dense aggregates of cells stain dark bluish purple. Normocellular particles observed in healthy adult horses consist of 50% fat and 50% cells (Fig. 15-2). Hypocellular particles consist mainly of fat (Fig. 15-3), and hypercellular particles are composed of dense cellular aggregates (Fig. 15-4). Hypocellular bone marrow particles suggest decreased hematopoiesis. In contrast, hypercellular particles suggest increased hematopoiesis (normal or abnormal response), infiltrative disease, or primary or metastatic neoplasia. Examination of the cellular composition of the marrow at higher magnification determines which of these conditions exists.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree