CHAPTER 7 Gastrulation, body folding and coelom formation
As was described in the previous chapter, blastulation results in the formation of the ICM, trophectoderm, epiblast, and hypoblast. The hypoblast and trophectoderm are extra-embryonic cell lineages that will participate in fetal membrane formation (see Chapter 9) whereas derivatives of the epiblast will found all of the embryonic cell lineages. Initially, this occurs through formation of three somatic germ layers from the epiblast: ectoderm, mesoderm and endoderm. A prominent derivative of the endoderm is the primitive gut, the formation of which lends its name to the entire process of germ layer formation – gastrulation, from the Greek term gastrula meaning small stomach. Besides the three germ layers, gastrulation also establishes the germ line, in the form of the primordial germ cells. Only the initial establishment of the germ line is covered in this chapter; further development of the primordial germ cells within the genital ridges is described in Chapters 4 and 15.
DEVELOPMENT OF THE AMNION
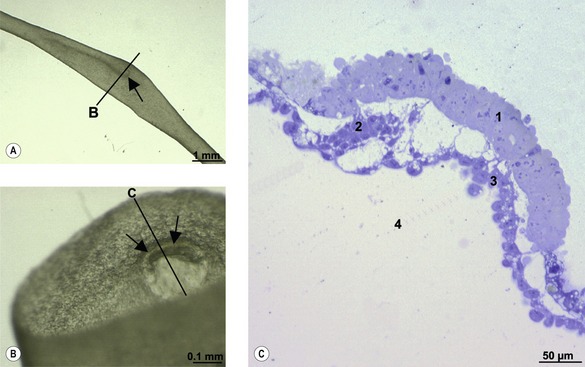
During the early phases of gastrulation, the trophectoderm is lined by a thin layer of extra-embryonic mesoderm (see below), the two layers together constituting the chorion. During gastrulation, the chorion forms folds – chorioamniotic folds – that surround the embryonic disc (Fig. 7-1). Gradually, the folds extend upwards to meet and fuse above the embryonic disc thereby enclosing the disc in a sealed amniotic cavity. The term amnion is generally used collectively for the cavity and its wall. The inner epithelium of the amnion originates from the trophectoderm and so, at the embryonic disc, it is continuous with the epiblast and later the embryonic surface ectoderm (see below). The outside covering of the amnion is composed of extra-embryonic mesoderm. Later, the amnion will become surrounded by yet another cavity, the allantois (see below and Chapter 9).
The site where the chorioamniotic folds meet and fuse is known as the mesamnion (Fig. 7-2). In the horse and carnivores, the mesamnion disappears leaving no connection between the amnion and chorion. As a result, foals, pups and kittens are born covered by an intact amnion which can be suffocating if not removed by the mother or an attendant. In contrast, in the pig and ruminants, the mesamnion persists; as a result, the amnion gets torn during parturition and offspring are generally born without covering membranes.
EARLY PHASES OF GASTRULATION
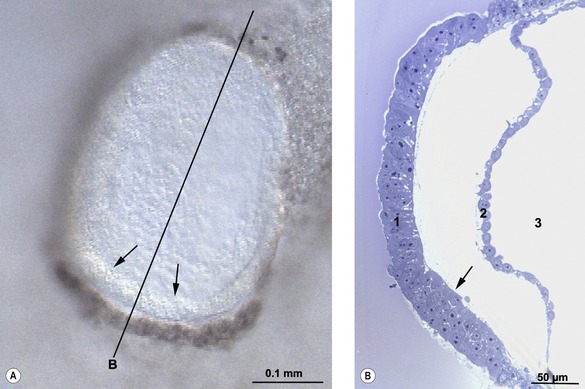
The onset of gastrulation has traditionally been linked to the morphological appearance of the primitive streak, an elongated accumulation of cells at the caudal pole of the future embryo proper. This structure is formed by epiblast cells accumulating posteriorly and gradually establishing a crescent-shaped thickening of the embryonic disc – the earliest morphological sign of the onset of gastrulation yet identified, at least in pigs and cattle (Figs 7-3, 7-4). The caudal crescentic thickening appears around Days 10 to 11 of gestation in the pig and around Days 14 to 15 in cattle. When the crescent-shaped thickening is established, ingression of epiblast cells to the space between the epiblast and hypoblast is initiated (Fig. 7-5). In laboratory animals, these early morphological signs of gastrulation in the epiblast are preceded by morphological and molecular changes in the hypoblast underlying the anterior pole of the epiblast (see Box 7-1 on molecular regulation).
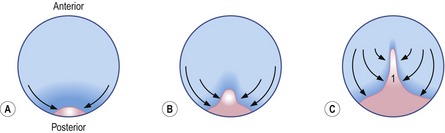
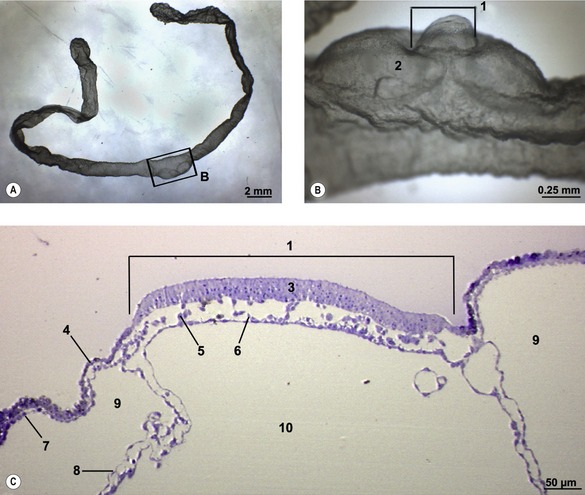
Fig. 7-3: Accumulation of epiblast cells in the posterior portion of the embryonic disc results in the formation of the primitive streak (1).
The epiblast cells constituting the posterior crescent soon gather in the embryonic disc midline to form the primitive streak (Fig. 7-3). In the streak, cells start to involute from the epiblast through its basement membrane to establish mes-endodermal precursors, i.e. cells capable of forming either mesoderm or endoderm. This is the first example of epithelio-mesenchymal transition during embryonic development, the process whereby cells change their characteristics from those of an epithelium (having strong intercellular connections) to become more loosely organized and, therefore, capable of migration. The term mesenchyme refers to loosely organized embryonic tissue regardless of germ layer origin. Interestingly, just as the initiation of gastrulation is marked by this epithelio-mesenchymal transition, so the cessation of this phase of development is marked by a suppression of this process.
INITIAL FORMATION OF THE MESODERM AND ENDODERM
The mes-endodermal precursor cells give rise to endoderm and mesoderm. The endoderm-forming cells integrate into, and displace, the hypoblast immediately beneath the epiblast, forming endoderm (Fig. 7-6). Other cells remain positioned under the epiblast and trophectoderm forming intra- and extra-embryonic mesoderm, respectively. The endoderm enlarges to form the upper lining of the primitive yolk sac under the epiblast and, at the margin of the embryonic disc, is continuous with the hypoblast (Figs 7-6, 7-7). The endodermally lined portion of the primitive yolk sac will later become enclosed within the embryo proper and develop into the primitive gut, whereas the hypoblast-lined portion of the cavity will be displaced extra-embryonically to form the definitive yolk sac (Fig. 7-8).
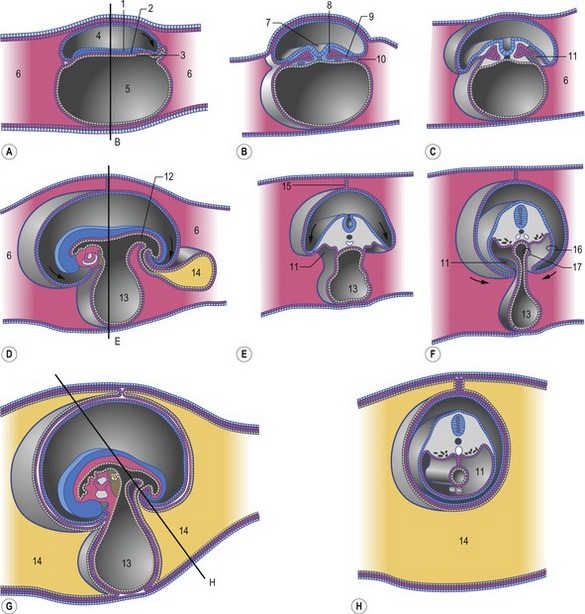
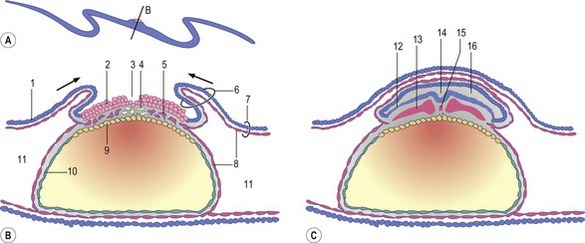
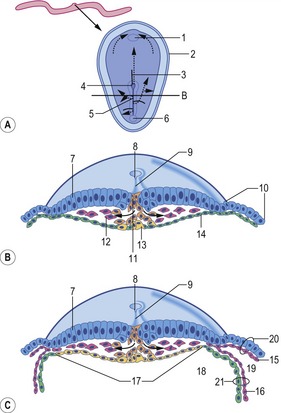
Fig. 7-8: Formation of the coelomic cavities. A, D, and G: Median sections of embryos at different stages of the cranio-caudal folding. Arrows indicate the direction of folding. B, C, E, F, and H: Cross sections of embryos at different stages of lateral folding. Arrows indicate the direction of folding. The section plane B is indicated in A, the section plane E is indicated in D, and the section plane H is indicated in G. 1: Ectoderm; 2: Mesoderm; 3: Endoderm; 4: Amniotic cavity; 5: Primitive yolk sac; 6: Extra-embryonic coelom; 7: Neural groove; 8: Paraxial mesoderm; 9: Intermediate mesoderm; 10: Lateral plate mesoderm; 11: Intra-embryonic coelom; 12: Hindgut; 13: Yolk sac; 14: Allantois; 15: Mesamnion; 16: Somatopleura; 17: Splanchnopleura.
Modified from Sadler (2004).
The development of the intra-embryonic mesoderm parallels that of the endoderm. It arises from a portion of the presumptive mes-endodermal cells that ingressed through the primitive streak but now remain in the space between the epiblast and the hypoblast. Formation of mesoderm is not limited to the area of the embryonic disc, however; mesodermal cells migrate far beyond the disc as the extra-embryonic mesoderm (Figs 7-6, 7-7). Intra- and extra-embryonic mesoderm each split into two sheets: one associating with the epiblast and trophectoderm to form the somatic or parietal mesoderm, the other associating with the endoderm and hypoblast as the visceral or splanchnic mesoderm (Figs 7-6, 7-7). Together, the trophectoderm and the extra-embryonic somatic mesoderm will form the outer layers of the embryonic part of the placenta, the chorion (see Chapter 9). The cavity forming between the somatic and visceral mesoderm is referred to as the coelom. Initially, the coelom is only located outside the embryonic disc and is therefore referred to as the extra-embryonic coelom or exocoelom. Soon, however, the split between the somatic and visceral mesoderm also involves intra-embryonic mesoderm portions, establishing an intra-embryonic coelom which, with the cranio-caudal and lateral foldings of the embryo, will later give rise to the body cavities (Fig. 7-8). When this happens, the somatic mesoderm gives rise to the parietal portions of the peritoneum and pleura, whereas the visceral mesoderm gives rise to the visceral portions of these serous membranes.
The intra-embryonic mesoderm is established in a posterior to anterior direction, in part progressing in parallel with the growing primitive streak. After involution, cells spread both laterally, forming the extra-embryonic mesoderm, and cranially, forming intra-embryonic mesoderm. Soon, the rate of growth of the entire embryonic disc overtakes that of the primitive streak, and the disc is lengthened into an initially oval, later pear-shaped, structure (Fig. 7-9). From extending well over half the length of the embryonic disc, the primitive streak gradually becomes more and more posteriorly located as a result of this differential growth.
Along with the relative posterior withdrawal of the primitive streak, a midline structure, the notochord, is laid down – a pivotal step in the establishment of the anterior-posterior embryonic axis (Figs 7-1, 7-6). The notochord is formed by epiblast cells ingressing through the primitive node, a specialized population of epiblast cells located at the anterior end of the primitive streak. The first cells that ingress through the primitive node form the prechordal plate, a mesodermal structure located just anterior to the tip of the notochord.
During the process of gastrulation, involution of cells forming the mesoderm and endoderm occurs through different organizer regions within the primitive streak. In the mouse, three such organizer regions have been identified: an early-gastrula organizer, contributing mainly to formation of extra-embryonic mesoderm; a mid-gastrula organizer, contributing mainly to endoderm and intra-embryonic mesoderm formation; and a late-gastrula organizer region giving rise to the prechordal plate and notochord. The late organizer region is the primitive node.
Anteriorly, the notochord is delimited by the prechordal plate, and anterior to this the epiblast is so tightly adherent to the newly formed endoderm that there is no space for intervening mesoderm (Fig. 7-6). This apposed epiblast and endoderm develops into the buccopharyngeal membrane which for a time seals the future opening between the oral cavity and the pharynx (see Chapter 14). Posteriorly, the notochord is delimited by a similar structure, the cloacal membrane, which for a time seals the common openings of the gut, urinary organs, and reproductive tract into the cloaca (see Chapter 14).