Chapter 208 Blood Gas and Oximetry Monitoring
INTRODUCTION
Although a thorough description of acid-base regulation and blood gas analysis is beyond the scope of this chapter (the reader is directed to several excellent references for a more detailed description of these subjects2-9), what follows is a brief overview of acid-base physiology and a practical method of interpreting blood gases (see Chapter 59, Acid-Base Disturbances).
BLOOD GAS ANALYSIS: GETTING STARTED
Step-by-Step Acid-Base Analysis
Number 1: Look at the pH
Table 208-1 shows normal arterial blood gas values in dogs and cats.14,15 As pH varies inversely with [H+], any process that increases H+ load will decrease pH and produce acidosis. Conversely, any process that decreases [H+] will tend to increase pH and produce alkalosis. The terms alkalemia and acidemia imply that blood pH is outside the normal range, which may or may not be true depending on the nature of the acid-base disorder and the effectiveness of organism’s compensatory mechanisms.
Table 208-1 Normal Arterial Blood Gas Values for Dogs and Cats14,15
| Dog | Cat | |
|---|---|---|
| Parameter | Value | Value |
| pH | 7.39 ± 0.03 | 7.39 ± 0.08 |
| PaCO2 (mm Hg) | 37 ± 3 | 31 ± 6 |
| PaO2 (mm Hg) | 102 ± 7 | 107 ± 12 |
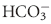 (mEq/L) (mEq/L) | 21 ± 2 | 18 ± 4 |
| Base excess (mEq/L) | −2 ± 2 | −2 ± 2 |
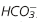 , Bicarbonate; PaCO2, partial pressure of arterial carbon dioxide; PaO2, partial pressure of arterial oxygen.
, Bicarbonate; PaCO2, partial pressure of arterial carbon dioxide; PaO2, partial pressure of arterial oxygen.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


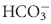 ) are present in the body in the range of milliequivalents per liter.
) are present in the body in the range of milliequivalents per liter.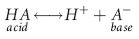
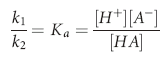
 ), and H+:
), and H+: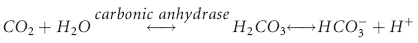
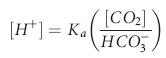
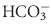 ), but also illuminated a process by which the body could buffer changes in H+ load, namely ventilation. Later Hasselbach would add further utility to the equation by substituting the partial pressure of CO2 in blood (PCO2) for dissolved CO2 and applying Sorenson’s concept of pH (the negative logarithm of [H+]) by putting the equation in logarithmic notation:
), but also illuminated a process by which the body could buffer changes in H+ load, namely ventilation. Later Hasselbach would add further utility to the equation by substituting the partial pressure of CO2 in blood (PCO2) for dissolved CO2 and applying Sorenson’s concept of pH (the negative logarithm of [H+]) by putting the equation in logarithmic notation: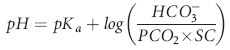
 (predominately extracellular buffer), the
(predominately extracellular buffer), the  system is of utmost importance. The reasons are twofold. Not only is the
system is of utmost importance. The reasons are twofold. Not only is the 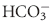 buffering system capable of responding to an acute change in [H+] within seconds of an acid load, but its role in the
buffering system capable of responding to an acute change in [H+] within seconds of an acid load, but its role in the 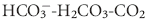 equilibrium equation allows changes in pH to be further modulated by changes in ventilation. This “open system” greatly enhances the buffering capacity of the
equilibrium equation allows changes in pH to be further modulated by changes in ventilation. This “open system” greatly enhances the buffering capacity of the 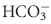 system and is capable of buffering changes in pH within minutes of an acid or alkali load. Finally, the kidneys play a major role in maintaining pH by increasing or decreasing acid elimination in the urine. This system takes hours to days to reach completion, but is the most capable of all the processes for returning the body’s pH to normal.
system and is capable of buffering changes in pH within minutes of an acid or alkali load. Finally, the kidneys play a major role in maintaining pH by increasing or decreasing acid elimination in the urine. This system takes hours to days to reach completion, but is the most capable of all the processes for returning the body’s pH to normal.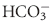 ). The pH varies directly with changes in the metabolic component and inversely with changes in the respiratory component. It would follow that pH changes produced by one component may be opposed by opposite changes in the other component. For instance, to compensate for a respiratory acidosis the organism will attempt to increase the concentration of
). The pH varies directly with changes in the metabolic component and inversely with changes in the respiratory component. It would follow that pH changes produced by one component may be opposed by opposite changes in the other component. For instance, to compensate for a respiratory acidosis the organism will attempt to increase the concentration of 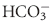 in the blood. The compensation may be strong, but rarely is it complete, and overcompensation does not normally occur.4
in the blood. The compensation may be strong, but rarely is it complete, and overcompensation does not normally occur.4