Chapter 118 Bleeding Disorders
PHYSIOLOGY OF HEMOSTASIS
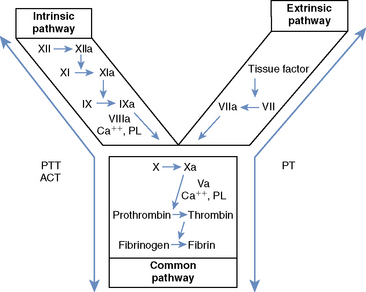
Secondary hemostasis comprises a series of enzymatic reactions, culminating in the cleavage of plasma fibrinogen to form fibrin, which stabilizes the primary hemostatic plug.1,3 All coagulation factors participating in secondary hemostasis are produced in the liver. Factors II (thrombin), VII, IX, and X are proenzymes that undergo a vitamin K–dependent modification before secretion from hepatocytes.1,3 Classically, two pathways of coagulation activation are recognized: an intrinsic and an extrinsic pathway (Figure 118-1). The intrinsic pathway is surface activated and operates strictly with the components present in the blood, whereas the extrinsic pathway requires a tissue factor for activation. These two pathways converge in a final common pathway of thrombin generation and fibrin formation. Defects of secondary hemostasis may be quantitative or qualitative coagulation factor disorders, or both.
EMERGENCY APPROACH TO THE BLEEDING PATIENT
Following sample collection, therapy should be initiated to stabilize the patient. Initial stabilization of the bleeding patient involves (1) control of hemorrhage, when possible; (2) blood transfusion, if anemia is significant; and/or (3) blood volume replacement, when hypoperfusion is present (see Chapters 65 and 66, Shock Fluids and Fluid Challenge and Transfusion Medicine, respectively). In animals with hemorrhagic shock, the most life-threatening problem is hypoperfusion. Initial therapy therefore should involve aggressive fluid therapy (isotonic crystalloids with or without synthetic colloid fluids) until blood is available. There is no justification for withholding fluid therapy in the anemic patient. Hypoperfusion will only exacerbate the tissue hypoxia.
DIAGNOSTIC APPROACH
Screening Laboratory Tests
Laboratory tests are essential to confirm and characterize the hemostatic defect (Table 118-1). These tests should be performed and interpreted carefully, along with the clinical findings, and with their individual limitations in mind. Normal values are listed in Table 118-2.
Table 118-1 Screening Tests for the Evaluation of Hemostasis
| Process | Screening Test | Component Evaluated |
|---|---|---|
| Primary hemostasis | Platelet enumeration* | Platelet numbers |
| Platelet estimation† | Platelet numbers | |
| Buccal mucosal bleeding time* | Platelet numbers and function, vascular integrity | |
| Secondary hemostasis | Activated clotting time† | Intrinsic and common pathways: factors XII, XI, IX, VIII, X, V, II, and fibrinogen |
| Partial thromboplastin time* | As with ACT, but more sensitive | |
| Prothrombin time* | Extrinsic and common pathways: factors III, VII, X, V, II, and fibrinogen | |
| Fibrinolysis | Fibrin split products* | Products of fibrinolysis or fibrogenolysis |
| D-Dimers* | Products of fibrinolysis; specific for lysis of cross-linked fibrin |
ACT, Activated clotting time.
Table 118-2 Normal Values for Screening Laboratory Tests of Hemostasis
| Test | Dog | Cat |
|---|---|---|
| Platelet count (× 103/μl) | 200 to 500 | 200 to 600 |
| Buccal mucosal bleeding time (minutes) | 1.7 to 4.2 | 1.4 to 2.4 |
| Cuticle bleeding time (minutes) | 2 to 8 | 2 to 8 |
| Activated clotting time (seconds) | 60 to 110 | 50 to 75 |
| Prothrombin time (seconds) | 6 to 11 | 6 to 12 |
| Partial thromboplastin time (seconds)* | 10 to 25 | 10 to 25 |
| Fibrin split products (μg/ml)* | <10 | <10 |
| D-Dimers (latex agglutination) (ng/dl) | <250 | <250 |
* Normal values are laboratory and technique dependent. Normal values for patient-side coagulometers are provided by the manufacturer.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree