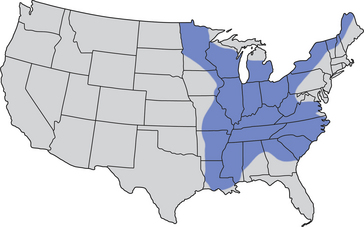
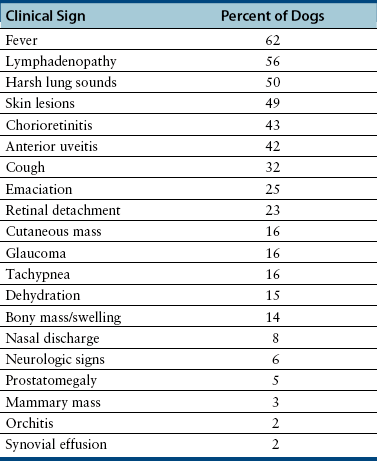
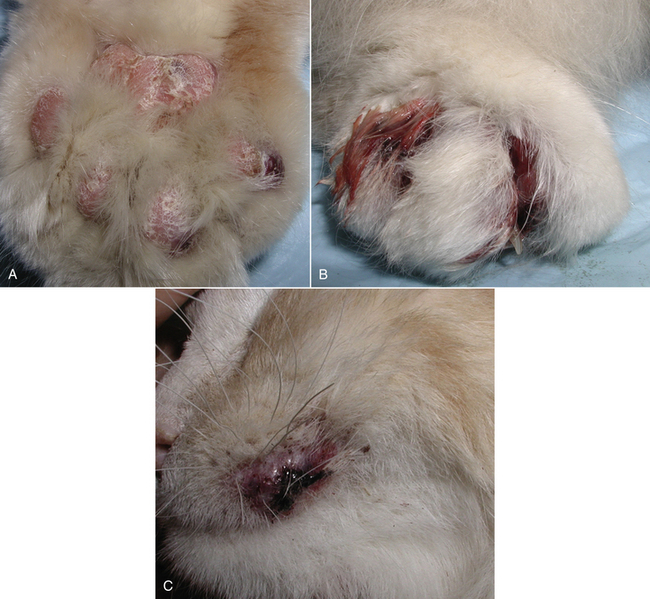
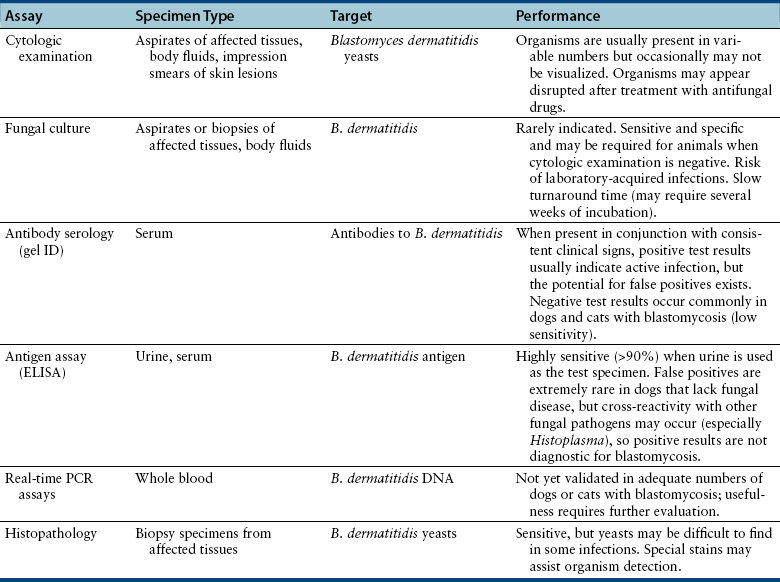
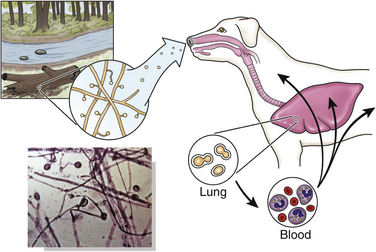
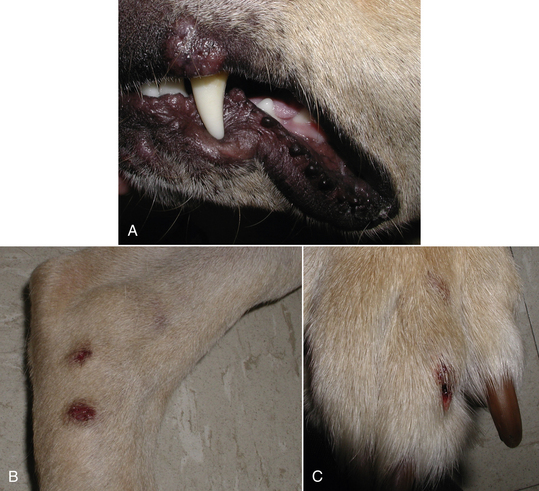
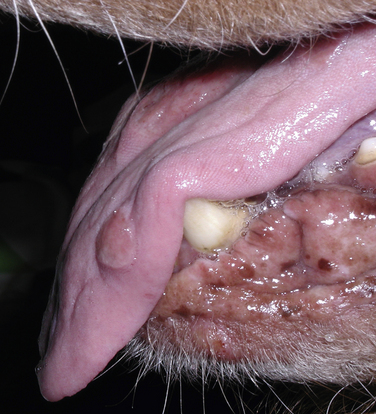
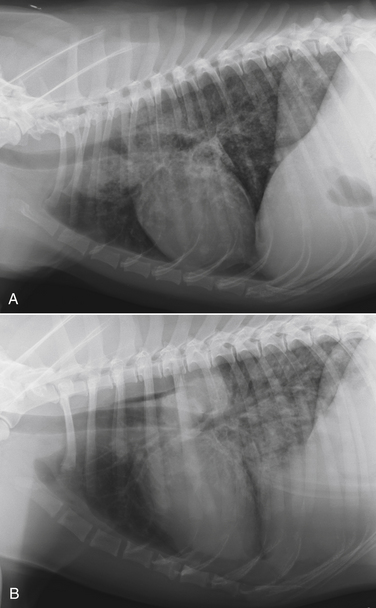
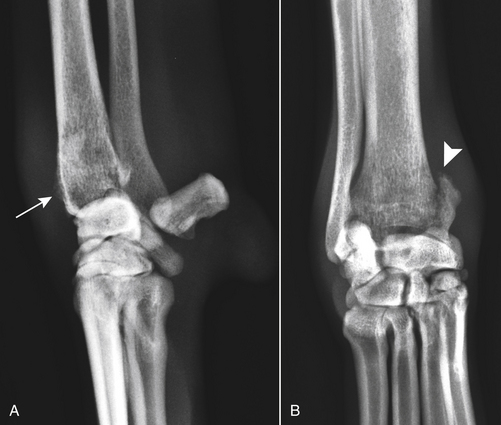
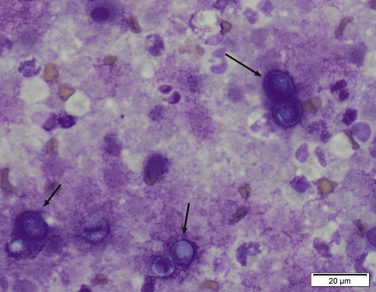
Chapter 60 Blastomyces dermatitidis is a dimorphic fungus that grows as a mycelial form in the environment and as a thick-walled budding yeast in tissues (at 37°C). Hyphae within soil produce conidia that are 2 to 10 µm in diameter. The conidia are thought to be aerosolized and inhaled by the host, where they transform into yeasts and produce a localized pulmonary or disseminated disease known as blastomycosis (Figure 60-1). The organism’s name (dermatitidis) stems from the fact that the skin is a common site to which the organism disseminates. FIGURE 60-1 Life cycle of Blastomyces dermatitidis. From the lung, the organism can disseminate to a variety of tissues, but especially the eye, lymph nodes, skin, and bones. B. dermatitidis is primarily found in the eastern parts of the United States, especially around the Great Lakes, along the Ohio and Mississippi river valleys, and in the southeastern states (Figure 60-2). A smaller focus of endemicity exists in the St. Lawrence river region in the northeast, which extends up into Ontario, Canada. Disease may also be seen in parts of Canada around the Great Lakes and has also been reported from Saskatchewan.2,3 Occasionally disease appears in nontraveled dogs that reside in non-endemic parts of the United States, such as in Wyoming and South Dakota. Canine blastomycosis has also been reported from India.4 Human blastomycosis occurs in Africa, but a different serotype is involved. Interestingly, reports of canine disease from Africa are absent from the literature. Blastomycosis was described in Europe in a dog with a travel history to the United States.5 The ecologic niche of B. dermatitidis appears to be warm, moist, sandy soil that is rich in organic debris such as decaying vegetation. However, this has not been thoroughly defined because the fungus is difficult to isolate from the environment. No consistent seasonality has been linked to disease. Genetic differences have been reported among isolates of B. dermatitidis within the United States. Using PCR-based typing methods, several different genotypes have been described (A, B, C, D, and E).6 Analysis by microsatellite typing suggests the existence of at least two genetically distinct groups, group 1 and group 2, the geographic distributions of which overlap.7 In human patients, group 2 isolates may be more likely to be associated with disease in older patients with comorbid conditions, and group 2 isolates are also more likely to be associated with dissemination than group 1 isolates.8 Young adult, large-breed (>15 kg) dogs are predisposed to blastomycosis, and a slight male predisposition has been identified in some studies.9 Overrepresented breeds include coonhounds, pointers, Weimaraners, Labrador retrievers, golden retrievers, and Doberman pinschers.9,10 This may reflect the increased likelihood of exposure of these dog types to organisms in the environment, because they are more likely to be used in outdoor activities such as hunting. Dogs younger than 6 months of age and as old as 17 years may be affected, but dogs in the 2- to 4-year age group have the highest risk of infection with the 4- to 6-year age group a close second.9,10 Residence less than 400 m from a body of water increased the risk of blastomycosis by a factor of 10 in one study from Louisiana,10 and exposure to sites of soil disturbance or excavation may also increase the risk of infection. In one report, B. dermatitidis was isolated from a woodpile near the Wisconsin River; over 14 years, 4 of 9 dogs housed in a kennel close to the woodpile developed blastomycosis.11 Most affected dogs are immunocompetent. Blastomycosis is rarely reported in cats from endemic regions of the United States and, as with other soil-borne mycoses such as histoplasmosis and cryptococcosis, blastomycosis can occur in cats that have been housed entirely indoors.12,13 In one study, 10% of 41 affected cats tested positive for FeLV antigen.14 Infection most often results from inhalation of conidia in the environment. Direct inoculation of organisms followed by localized cutaneous disease and/or osteomyelitis (inoculation blastomycosis) occurs rarely in dogs and human patients.15,16 Once within the host, the conidia transform into yeasts that can resist destruction by neutrophils. The yeasts trigger a pyogranulomatous inflammatory response. Important virulence factors include a cell surface glycoprotein known as BAD-1 (previously known as WI-1), and other cell wall components such as α-1,3-glucan and possibly melanin.17,18 BAD-1 is an adhesin that binds to host cell receptors on macrophages. It may also contribute to the yeast’s ability to evade the host immune response by influencing cytokine secretion and impairing complement activation.17,19 In some animals, the organism spreads hematogenously from the lungs to associated lymph nodes and other extrapulmonary sites. Extrapulmonary sites of predilection include the skin, eye, bone, reproductive tissues (prostate and testes), and the central nervous system (CNS). However, virtually any tissue in the body may be affected, and infection has been reported in the mammary glands, nasal or oral cavities, cardiac tissues (myocardium, endocardium, and pericardium), and very rarely in the kidneys, liver, spleen, peritoneum, gastrointestinal tract, and urinary bladder.20 Clinical signs are variable. Dogs may develop subclinical infections, acute or chronic disease that is localized to the lungs and associated lymph nodes, or severe and progressive signs that result from dissemination of the organism to multiple extrapulmonary sites. The incubation period is variable and not precisely known, but is estimated to range from 5 to 12 weeks. Nonspecific signs of illness such as lethargy, weakness, fever, inappetence, and weight loss are common (Table 60-1). Other common clinical manifestations (between 20% and 50% of affected dogs) include signs of respiratory involvement (such as cough and increased respiratory rate), lymphadenopathy, ocular manifestations, cutaneous lesions, and lameness.10,21 Lameness may result from fungal osteomyelitis, arthritis, or rarely, hypertrophic osteopathy.22 Gastrointestinal signs (vomiting, diarrhea, hematemesis, and melena); polyuria or polydipsia; mammary gland masses; laryngeal masses; nasal discharge, sneezing and/or epistaxis; testicular masses; dysuria or hematuria secondary to prostatic involvement; cardiac arrhythmias; and neurologic signs due to meningoencephalitis or ependymitis occur less frequently.10,23–29 Neurologic involvement secondary to extension of intranasal or retrobulbar Blastomyces granulomas through the calvarium can also occur.25,29 Rarely, thromboembolic disease has been reported.30 In cats, clinical signs of blastomycosis are similar to those in dogs, although neurologic and gastrointestinal involvement may be more prevalent.31 Physical examination abnormalities in dogs with blastomycosis commonly include fever, thin body condition, dehydration, and signs of respiratory involvement. Fever is present in approximately 50% of affected dogs and is usually low grade (103°F to 104°F or 39.4°C to 40.0°C) but occasionally exceeds 106°F (41.1°C).20,27 Respiratory signs include cough, tachypnea, increased respiratory effort, and increased lung sounds on thoracic auscultation. Other common findings on physical examination are firm cutaneous or subcutaneous masses, draining or ulcerated skin lesions, and peripheral lymphadenopathy. Careful palpation of the entire skin surface can reveal small skin lesions. Cutaneous and subcutaneous lesions are frequently found on the trunk, limbs, and digits and can also involve the muzzle (Figure 60-3). Lesions of the tongue, gingiva, or mucocutaneous junctions may also be present (Figure 60-4). Facial swelling, exophthalmos, or facial deformity has been described in some affected dogs.25,32 Dogs with nasal cavity involvement can have stertorous respiration, decreased nasal airflow, or nasal discharge. Ocular signs may be unilateral or bilateral and include chorioretinitis (sometimes with retinal detachment); lesions consistent with optic neuritis; endophthalmitis or panophthalmitis; uveitis with aqueous flare, iris bombé, synechia, and miosis; cataract formation; conjunctivitis; keratitis; and photophobia.33,34 Severe ocular involvement may result in increased intraocular pressures and/or loss of vision. Dogs with musculoskeletal involvement may be lame, have firm swellings associated with long bones, or exhibit joint swelling, warmth, and/or pain. Dogs with testicular blastomycosis may have scrotal swelling or palpable testicular masses. Neurologic signs are present in fewer than 5% of affected dogs and can include seizures, hypermetria, decreased placing reactions, tetraparesis, circling, ataxia, blindness, decreased or absent menace and/or pupillary light reflexes, nystagmus, and decreased facial sensation. FIGURE 60-3 Disseminated blastomycosis in a labrador retriever. Nodular cutaneous lesions are present on the muzzle (A). There are also multiple, ulcerated, and draining skin lesions on the distal limbs (B) and digit (C). (Courtesy Dr. Sheila Torres, University of Minnesota.) FIGURE 60-4 Nodular and ulcerated lesions of the tongue in a dog with disseminated blastomycosis. (Courtesy Dr. Sheila Torres, University of Minnesota.) Physical examination findings in cats with blastomycosis are similar to those described in dogs. These include fever, thin body condition, peripheral lymphadenopathy, tachypnea, increased or decreased lung sounds, ocular abnormalities (chorioretinitis with retinal detachment, uveitis, panophthalmitis, and secondary glaucoma), nodular or draining skin lesions (Figure 60-5), and a variety of neurologic signs that include obtundation, ataxia, pelvic limb paresis, circling, hyperesthesia, decreased placing reactions, and blindness.13,31,35–37 A diagnosis of blastomycosis is usually suspected based on the presence of suspicious clinicopathologic abnormalities in a dog or cat from an endemic area. The diagnosis is usually confirmed through cytologic examination of fine needle aspirates of affected tissues (especially skin lesions and lymph nodes), impression smears of draining skin lesions, respiratory lavage specimens (transtracheal washes or bronchoalveolar lavage), or body fluids (especially CSF or ocular fluid, but also synovial fluid or urine sediment). Other methods of diagnosis include histopathology (e.g., of bone or tissue biopsies), fungal culture, and PCR-based assays (Table 60-2). Serologic assays that detect antigen and antibody are also available, but when used alone, these do not confirm a diagnosis of blastomycosis. The hemogram of dogs or cats with blastomycosis can be unremarkable. However, a mild, normocytic, normochromic nonregenerative anemia is present in many affected animals. Mild to moderate neutrophilia is also present in many dogs and may be accompanied by a mild to moderate bandemia.10,38 Mild monocytosis, lymphocytosis, or lymphopenia may be detected. Serum biochemistry findings in animals with blastomycosis include mild to moderate hyperglobulinemia due to a polyclonal gammopathy, hypoalbuminemia, and, uncommonly, mild hypercalcemia.10,38 Hypoalbuminemia was present in 70 of 91 (77%) of dogs in one study, hyperglobulinemia in 58%, and hypercalcemia in 14% of dogs.10 Radiographic patterns in dogs with blastomycosis vary considerably and include unstructured, miliary, or nodular interstitial patterns; a bronchointerstitial pattern; alveolar or mixed alveolar-interstitial patterns; pulmonary mass lesions (>3 cm in diameter); or single or multiple large (0.5 to 2.9 cm) pulmonary nodules that can resemble metastatic pulmonary neoplasia (Figure 60-6).10,40,41 Tracheobronchial lymphadenopathy is present in approximately 25% of dogs.40 Pleural thickening and mild pleural effusion are uncommonly identified (≤10% of affected dogs). Focal bronchiectasis has also been described. In one study, only 2 of 125 dogs with blastomycosis had no visible abnormalities on thoracic radiography. Findings in affected cats include diffuse miliary or nodular interstitial patterns, lobar consolidation, and/or pleural effusion.13,31 FIGURE 60-6 Radiographic patterns in pulmonary blastomycosis. A, Lateral thoracic radiograph from a 2-year-old female spayed Labrador retriever with a 1-week history of cough and inappetence. A diffuse miliary nodular interstitial pattern is present. B, Lateral thoracic radiograph from a 5-year-old female spayed German shepherd dog with lethargy and inappetence. An alveolar pattern is present in the caudal lung lobes, along with marked hilar lymphadenopathy. There is also a nodular lesion in the cranial lung lobes just ventral to the trachea. (Courtesy Daniel Cronk, University of Minnesota.) Radiographs of affected bone typically show osteolysis, often accompanied by periosteal proliferation and soft tissue swelling. Pathologic fractures may be identified (Figure 60-7). Evidence of hypertrophic osteopathy was present bilaterally along the humerus, radius, ulna, metacarpi, and phalanges of a dog with a mass lesion in the right middle lung lobe.22 FIGURE 60-7 Lateral (A) and anteropalmar (B) radiograph of the right carpus showing an osteolytic and osteoproductive (small arrow) lesion with a pathologic fracture (arrowhead) and associated soft tissue swelling in the distal radius of a 6-year-old male neutered Labrador retriever with disseminated blastomycosis. (Image courtesy Daniel Cronk, University of Minnesota.) Computed tomographic findings in dogs with CNS blastomycosis include intra-axial, intranasal, or retrobulbar mass lesions that are uniformly or heterogeneously contrast enhancing.25 An intra-axial contrast-enhancing mass lesion was also described in a cat.42 Intranasal or retrobulbar mass lesions can invade the cribriform plate or other parts of the skull, with associated osteolysis. Meningeal or periventricular contrast enhancement may also be present.25,26 MRI findings have not been extensively described. One dog had a retrobulbar mass lesion that extended through the calvarium; the lesion was iso- to hypointense on T2-weighted images, slightly hypointense on T1-weighted images, and showed strong homogenous contrast enhancement.29 Cytologic examination of fine-needle aspirates, respiratory wash specimens, or body fluids frequently reveals large numbers of B. dermatitidis yeasts. The yeasts are 8 to 15 µm in diameter, have a thick, refractile cell wall, and exhibit broad-based budding (Figure 60-8). Daughter cells are nearly as large as the parent cell when they detach. A pyogranulomatous inflammatory response is usually present. Occasionally, organisms are not seen; the sensitivity of transtracheal lavage for diagnosis of pulmonary blastomycosis in two studies of 17 and 39 dogs was 76% and 69%,38,41 whereas the sensitivity of fine-needle aspiration of the lung was 46 of 57 (81%).38 FIGURE 60-8 Cytology of a fine-needle aspirate of a skin lesion from a dog with blastomycosis. Blastomyces yeasts (arrows) have a thick wall and exhibit broad-based budding. A pyogranulomatous inflammatory response is also present. (Image courtesy Dr. Jed Overmann, University of Minnesota.)
Blastomycosis
Etiology and Epidemiology
Clinical Features
Physical Examination Findings
Diagnosis
Laboratory Abnormalities
Serum Biochemical Tests
Diagnostic Imaging
Advanced Imaging
Microbiologic Tests
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Blastomycosis
Only gold members can continue reading. Log In or Register to continue