Fig. 1
Biosafety management program core elements
The purpose of the Biosafety Program is to prevent or minimize employee exposures or the accidental environmental release of hazardous biological agents through the promotion of safe laboratory practices and procedures and proper use of containment equipment and facilities by employees, students, visitors, and contractors.
The biosafety program vision and mission for maximum effectiveness should be able to directly link to the institution’s vision and mission statements or goals. An institution’s biosafety policy is especially critical in that it defines the roles and responsibilities for the program at all levels, as well as the methodology for defining program goals and how individual accountability at all levels is measured. It is important that the biosafety policy clearly states the intent and direction of institutional leadership, and supporting documentation must identify a chain of responsibility for information on biosafety-related issues. It is important to note that the written biosafety/biocontainment policy is just another mechanism of communicating the policy to the employees. Laboratory supervisors’, principal investigators’, biosafety and safety professionals’, and other institutional leaders’ attitude toward biosafety and what they do both as individuals and collectively, or fail to do, is a more powerful expression of the actual biosafety policy and culture than the actual written words of the policy. In other words, a strong biosafety culture will only be created and maintained when all levels of management demonstrate their commitment personally by embodying and rewarding culture-supportive behaviors and conclusively addressing behaviors in others that undermine the culture of safety.
Following clockwise on the diagram of Fig. 1, the fourth through seventh balloon collectively represent universal employee participation through hazard identification and assessment, hazard prevention and control, information and training, and evaluation of program effectiveness. Employee participation is critical to the success of any biosafety program. Employees from all levels within the organization need to be engaged and provided opportunities to actively participate within the biosafety program, such as participating on various safety committees engaged in risk assessment and policy development, participating in active discussions of biosafety topics at regularly scheduled laboratory meetings, inclusion of lab staff on biosafety inspection and audit teams, and, finally, ensuring that employees are involved in determining biosafety-related goals and metrics for their specific work areas. Organizations need to ensure that employees are actively encouraged to report unsafe conditions, accidents, and near misses, as well as making recommendations to improve safety and health within their working environment. The critical components of this encouragement are the creation and enforcement of “whistle-blower”-type protections for those employees that report concerns at a minimum and ideally the creation of a reward structure for reports that encourage safe practices but discourage false or retaliated reporting. As a safety culture is established within an organization and employees adopt those critical safety behaviors as the norm, an environment is created in which risky behaviors are no longer tolerated among peers or management [2]. Of course, any robust biosafety program must include a continuous formal process of hazard identification, risk assessment, hazard prevention, and mitigation, which is conducted by the laboratory supervisor or a committee such as a biosafety committee. We will further discuss these elements in the next section as they are closely related.
3 Risk Assessment for Veterinary Diagnostic Labs
The nature of veterinary diagnostic work, which involves working with samples of unknown status that may contain strict veterinary pathogens or zoonotic pathogens, differs from work conducted in research laboratories where the agent risks are known and can be incorporated into the initial risk assessment. Diagnostic workers who work in (human) public health have a significant and known occupational exposure risk to cultures or tissues containing pathogens that infect humans. However, the risk to those individuals working in veterinary diagnostic laboratories for occupational exposure to pathogens that can infect and cause disease in humans is not negligible. Indeed 65 % of identified infectious diseases in humans are caused by organisms which infect multiple hosts in nature, and 75 % of emerging human infectious diseases over the past three decades have been zoonotic in nature [3]. Accordingly, veterinary health professional and diagnostic workers should be familiar with the principles and practices for preventing transmission of infectious agents (collectively known as “standard precautions”). In 2006, the National Association of State Public Health Veterinarians’ (NASPHV) Veterinary Infection Control Committee published a compendium of veterinary standard precautions, Zoonotic Disease Prevention in Veterinary Personnel [4], which is an excellent review of the standard precautions one should employ while working with animals in the field, veterinary clinic, and diagnostic labs that handle animal tissues or zoonotic agents. Another excellent reference on standard precautions entitled Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings was published in 2007, by the Healthcare Infection Control Practices Advisory Committee (HICPAC) [5]. This guide discusses worker practices and precautions to minimize infectious disease transmission in patient care and diagnostic labs and is another valuable reference for those individuals working in both animal and human diagnostic laboratories.
In addition to occupational risks associated with potentially zoonotic veterinary pathogens, those individuals conducting risk assessment with veterinary pathogens in which humans are not a relevant host must be cognizant of those risk drivers for animal agriculture that are based primarily on the potential economic impact on animal health associated with the release of a veterinary pathogen, as well as the international trade implications of disease [6]. Some agent-specific characteristics that should be evaluated as part of a comprehensive risk assessment involving pathogens of veterinary significance are the following:
Is the agent endemic or foreign to the region?
Is the host animal native or exotic to the area?
What is known regarding the morbidity and mortality caused by the agent, including whether it is exclusively an animal agent or a zoonotic agent?
Are there effective prophylaxes, treatments, or vaccines available (for animals and/or humans)?
What are the shedding and transmission patterns of the agent in the relevant host species?
Will the agent be introduced into species for which there are no data?
Are there active control or eradication programs for the disease?
What are the environmental stability, quantity, and concentration of the agent?
How will the agents be used—in animals (large or small scale), in the field, or in the laboratory?
What is the host range of the agent and are there ongoing surveillance testing programs?
Is the agent vector-borne and transmitted, and what is the occurrence of competent vectors? [7].
A risk assessment must also review the operational elements of the proposed work with biological hazards. Diagnostic laboratories need (at a minimum) to conduct risk assessment on the following operational areas:
Receiving, unpacking, and transfer into the laboratory.
Initial processing of the sample.
Preparation of sample for analysis (culture, ELISA, polymerase chain reaction (PCR), etc.) [8].
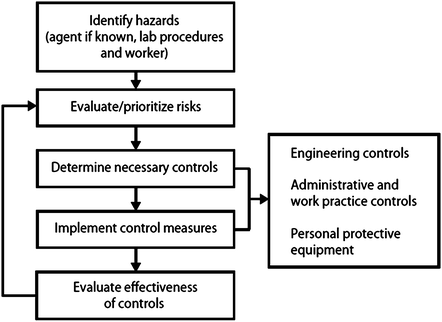
Although risk assessment methodology can be either quantitative or qualitative, it is recognized that most life science institutions favor qualitative methodology such as operational risk management, which is a continuous process that can be broken down into the key steps depicted in the diagram of Fig. 2. For more detailed information on operational risks associated with specific diagnostic activities, the authors recommend that the readers review US Centers for Disease Control and Prevention (CDC): Guidelines for Safe Work Practices in Human and Animal Medical Diagnostic Laboratories (www.cdc.gov/mmwr/preview/mmwrhtml/su6101a1.htm) [9]. Finally, a risk assessment must review the training, experience, and competency of the individuals who will be conducting the work with biohazards. The risk for exposures, laboratory-acquired infections, and the unintended release of research, clinical, or diagnostic materials to the environment should ultimately be reduced by ensuring the competency of laboratory workers at all levels. Indeed, many institutions have successfully implemented a formal training and mentoring program for individuals new to the laboratory regardless of previous education and experience elsewhere, and the authors believe this is a practice that should be encouraged at all laboratories. However when working with a new biohazard or a change to work currently performed, the level of risk changes and an evaluation of the risk must be conducted, which includes a review of the experience and competency of the laboratory supervisor and staff as part of this formal risk assessment process. An MMWR supplement Guidelines for Biosafety Laboratory Competency was published on behalf of CDC and the Association of Public Health Laboratories (APHL) in 2011 [10]. These competencies outline the essential skills, knowledge, and abilities required for working with biologic agents at the three highest biosafety levels (BSLs) (levels 2, 3, and 4) as defined in the HHS/NIH publication Biosafety in Microbiological and Biomedical Laboratories, 5th edition [11]. This document can be a useful tool in categorizing the level of laboratory competency of staff in a diagnostic laboratory as well as for the development of competency-based biosafety training programs.