References
Drug
Treatment
Withdrawal
perioda
Measured
variables
Subjects
Behavioral or
neurochemical outcome
Berger et al.
(142)
COC
Exposure to COC cues with or without oral 4 mg/kg haloperidol
Unknown
Plasma HVA, ACTH, and cortisol; craving and anxiety measured in response to visual cue exposure
Male COC-dependent inpatients
Increases in anxiety, craving, ACTH, cortisol, and HVA following cue exposure; increases in anxiety, and craving attenuated by haloperidol
Boileau et al.
(143)
AMP
On days 1, 3, 5, then after 2 weeks and 1 year, subjects received 0.3 mg/kg AMP or placebo p.o.; PET scans were performed on day 1, 2 weeks and 1 year; all treatments were given in the same context
2 weeks,1 year
Striatal DA release; behavioral and psychomotor responses
Healthy males without a history of substance abuse
Greater psychomotor response (vigor and eye-blink rates) and increased DA release observed at 2 weeks and 1 year, as compared to day 1
Boileau et al.
(144)
AMP
Days 1, 3, and 5 subjects received 0.3 mg/kg AMP p.o.; ∼2 weeks later, placebo was administered p.o. in the same context; PET scans were performed on day 1 after AMP and at 2 weeks after placebo designed to look like AMP
2 weeks (for most subjects)
Striatal DA release; behavioral and psychomotor responses
Healthy males without a history of substance abuse
AMP increased DA release in the VS under both AMP and placebo conditions; both AMP and placebo increased subjective ratings of euphoria, etc.
Breier et al. (145)
AMP
Subjects received 0.2 mg/ kg AMP i.v.; PET scans were performed during the treatment
NA
Striatal DA release (PET); behavioral and psychomotor responses
Healthy vs. schizophrenic male and females without a history of substance abuse
AMP increased striatal DA in both healthy and schizophrenic subjects, but to a greater extent in schizophrenics
Cox et al.
(146)
COC
1 mg/kg intranasal COC or placebo
Unknown
Striatal DA release; behavioral and psychomotor responses
Male and female non-dependent
COC users
Number of past COC/AMP experiences was positively correlated with DA increase in VS
Foltin et al.
(147)
COC Base (Crack)
Smoked COC twice/day for 3 consecutive days; escalating dose group (12 mg × 1, 25 mg × 1, 50 mg × 4) vs. fixed dose group (50 mg × 6)
2 weeks
Physiological measures (heart rate, blood pressure); subjective drug ratings
Male, non-treatment seeking COC users
Escalating dose group displayed dose-dependent increases in all dependent variables; no changes in fixed group
Johanson and Uhlenhuth (148)
AMP
Alternating p.o. 5 mg AMP vs. placebo over 4 days; drug order was counterbalanced and pill color indicated AMP or placebo; on days 5–9, subjects chose either AMP or placebo; this sequence of events occurred three times in the same subjects
None
Subjective drug effects (POMS, vigor, and elation)
Healthy men and women without a history of substance abuse
AMP increased vigor, elation, arousal, and positive mood similarly across each of the three sessions; however, preference for AMP declined over the three sessions
Kegeles et al. (149)
AMP
Two i.v. administrations of 0.3 mg/kg AMP spaced 16 days apart; SPECT scan was performed during each treatment
16 days
Synaptic DA release
Healthy males without history of stimulant use
AMP elicited a similar within-subjects DA increase and subjective activation across the two sessions (i.e., no sensitization)
Kelly et al.
(150)
AMP
15 day in-house study, counterbalanced for drug exposure order; two oral doses per day of 0.14 mg/kg AMP in 10% EtOH for 3 consecutive days; 3 days of twice daily 6% EtOH “placebo” treatment separated a second period of 3 more active days of drug treatment
None
Subjective effects, food intake, accuracy on some work tasks, verbal interaction, and cigarette smoking
Healthy males
AMP decreased food consumption and increased task performance, verbal interaction, and cigarette smoking similarly in both sessions; AMP increased ratings of potency, liking, stimulated anxious, and sedated vs. placebo in the first session; tolerance developed to these effects in the second drug exposure session
Leyton et al. (151)
COC
Intranasal COC 3.0 mg/kg on day 1, and then three doses of 0.6, 1.5, and 3.0 mg/kg COC on each of the following 3 consecutive days. Effects of pre-treatment with an amino acid mixture and of l-dopa/carbidopa on COC effects were also evaluated
None
Subject drug effects; subjective visual COC cue response
Male, non-treatment seeking COC users
COC dose-dependently increased euphoria ratings. l-dopa/carbidopa treatment reduced COC- and COC cue-induced craving. Frequency of past year COC use predicted the cue-induced item “want cocaine;” data for each day were not shown separately, not permitting evaluation of possible sensitization
Martinez et al.-Exp. 1 (152)
AMP
Subjects received 0.3 mg/kg AMP i.v.; PET scans were performed during the treatment
At least -14 days before study initiation
DA release in striatal subregions; vital signs; subjective effects
Male and female healthy controls and COC-dependent users
COC-dependent users had blunted DA responses in the striatum and rated AMP as less euphoric than healthy controls
Martinez et al.-Exp. 2 (152)
COC
Three sample sessions: users self-administered 0, 6, or 12 mg COC. Three choice sessions: users received a priming dose of COC and were given the choice to self-administer 0, 6, or 12 mg COC or receive $5
At least – 14 days before study initiation
Subjective effects; number of times COC was chosen over money (from 0 to 5)
COC-dependent users
Users rated 12 mg COC as the most euphoric; users chose the 12 mg dose of COC over money 3.3 out of 5 times; magnitude of decrease in AMP-induced DA response in the striatum in Exp. 1 was inversely correlated with likelihood of choosing COC over money
Nagoshi et al.
(153)
COC
Initial test doses were placebo and 10–60 mg/kg COC; retest doses were placebo and 40 mg/kg COC
1–3 weeks
Cardiovascular and subjective responses
Male i.v. COC users
Heightened heart rate for both the placebo and common 40 mg/kg COC dose on retest compared to initial response; similar subjective responses to the 40 mg/kg COC dose were found across time (no sensitization)
Newlin and Thomson (154)
EtOH
Three identical sessions of oral 0.5 g/kg of EtOH followed by a fourth placebo session
None
Heart rate, finger temperature, finger pulse amplitude, skin conductance, and general motor activity
Healthy male college students with or without a parental history of alcoholism
Greater motor activity across sessions in sons of alcoholics; sensitization to finger pulse amplitude; and lack of tolerance to changes in skin conductance and finger temperature in sons of alcoholics
Newlin and Thomson (155)
EtOH
Four identical sessions of oral 0.5 g/kg of EtOH followed by a fifth placebo session
None
Heart rate, pulse transit time, finger temperature, cheek temperature and body sway
Healthy male college students with or without a parental history of alcoholism
Sensitization to pulse transit time and body sway in sons of alcoholics
Rothman et al.(156)
COC
Day 1: i.v. saline or 40 mg/kg COC in two contexts; day 2: i.v. saline or 25 mg/kg COC in “test” context-only
None
Physiological and subjective responses; hormone levels
Healthy male and female subjects with a history of i.v. COC use (at least three times in the month prior to admission)
No conditioned or unconditioned sensitization to COC; acute COC increased cortisol
Sax and
Strakowski (157)
AMP
Placebo and 0.25 mg/kg AMP were administered on alternating days so that each subject received three doses of AMP separated by 48-h intervals; order of treatment was randomized
None
Eye-blink rate, mood, motor activity/energy, speech level, subjective drug effects, TPQ
Healthy male and female subjects without a history of alcohol or drug abuse
Greater change in elevated mood between AMP doses 1 and 3 (sensitization) was associated with higher rating of novelty seeking on the TPQ abuse
Strakowski and Sax (158)
AMP
Placebo and 0.25 mg/kg AMP were given on alternating days so that each subject received three doses of AMP separated by 48-h intervals; order of treatment was randomized
None
Eye-blink rate; vigor, talking ease; subjective ratings of euphoria, vigor, and drug-liking; TPQ
Healthy males and females without a history of stimulant use or abuse
Progressively increased subjective responses and eye-blinking following repeated AMP administration (sensitization)
Strakowski et al. (159)
AMP
Placebo and 0.25 mg/kg AMP were given on alternating days so that each subject received two doses of AMP separated by a 48-h interval; order of treatment was randomized
None
Eye-blink rate and clinician-rated scales for manic symptoms
Healthy males and females without a history of stimulant use or abuse
Increased eye-blink rate, ratings of energy level, mood and talkativeness following the second AMP administration (sensitization) compared to the first
Strakowski et al. (160)
AMP
Treatments were given on days 1, 3, and 5; subjects were given placebo on all 3 days, 0.25 mg/kg AMP on all 3 days, or placebo on days 1 and 3 then 0.25 mg/kg AMP on day 5
None
Eye-blink; subjective ratings of vigor and euphoria
Healthy males and females without a history of stimulant use
Decreased drug liking in the repeated AMP, compared to single AMP administration group. Sensitized vigor rating in the group that received three administrations of AMP, compared to the group that received one, in females only
Szechtman et al. (161)
APO
12 s.c. injections of 0.0107 mg/kg APO every 2 weeks
None
Yawning, growth hormone, and drug-induced nausea
Healthy males without a history of substance abuse
Sensitization to yawning occurred, shown as shorter latency to onset, and an increase in peak activity
Volkow et al. (162)
COC
PET scan was performed in the presence of radiotracer [11C]raclopride; exposure to neutral or COC cues
None
DA level changes inferred from occupancy of dopamine D2-like receptors; COC craving questionnaire; addiction severity index; COC selectivity assessment scale
Male and female COC-dependent users
Individual differences in cue-induced DA changes correlated with differences in subjective drug craving; high scores on measures of withdrawal symptoms and addiction severity were associated with larger striatal dopamine changes in response to COC cues
Wong et al.
(163)
COC
PET scan was performed in the presence of radiotracer [11C]raclopride; exposure to neutral or COC cues
24 h before study initiation
DA level changes inferred from occupancy of dopamine D2-like receptors; subjective visual cue responses
Male and female COC-dependent users
Increased cue-induced craving was associated with increased D2 receptor occupancy in putamen
Before sensitization-related theories of addiction became acknowledged in the addiction research field, a common assumption was that repeated drug exposure would lead to tolerance to the majority of the behavioral drug effects. However, clinicians noted that some motor responses to psychostimulants did not follow a tolerance-like profile. With amphetamines, for instance, clinicians observed that their repeated abuse induced prolonged increases in stereotypies (repetitive movements) and psychotic-like, paranoia-related episodes (165, 166). More recently, several systematic studies of behavioral sensitization in humans have shown that the repeated intermittent administration of amphetamine can produce persistent potentiation of eye-blink responses, drug-cue-biased attention, and also increases in subjective euphoria (143, 158, 159). There are data, however, indicating that subjective ratings of euphorigenic or “pleasurable” drug effects might not be the most appropriate dependent variable to test sensitized responses (activation or vigor ratings have shown results that are more consistent with sensitization theories). Some studies indicate that drug abusers, in particular psychostimulant abusers, describe increases in drug-seeking behavior even when they show tolerance to the euphorigenic effects of the drug or have decreased drug liking (96). Also, as mentioned before, accumulating evidence suggests that the “pleasurable” or rewarding effects of drugs might not be mediated by DAergic mechanisms (those that undergo enduring drug-induced sensitization). Accumbens DA functions appear to be more closely related to motivation, activation, vigor, and salience of reinforcers and reinforcer-associated cues than to drug-induced positive emotional responses or pleasure (92, 93, 167–169). Indeed, self-reports of sensitized vigor and energy levels have been described in studies with repeated d-amphetamine administration in subjects with past drug exposure, but no history of substance dependence (143, 160); the same results were found when vigor and energy levels were rated by clinicians (158, 159). Interestingly, in some of those studies, sensitized behavioral responses to amphetamine were found in the absence of an increase in drug liking; in fact, self-reported drug liking was either not altered or decreased with repeated drug exposure in these studies (158, 160).
The majority of human studies that have found behavioral sensitization to psychostimulants were conducted in healthy subjects, without a drug abuse history. Although at face value this sounds similar to how animal research is conducted – with drug-naïve subjects – there are important differences. Because, for ethical reasons, it is rarely possible to justify the use of completely drug-naïve subjects in drug exposure research, when subjects were naïve to the particular drug being studied, they were not naïve to other drugs, some of which have been demonstrated to induce cross-sensitization to the study drug. This raises the question of what the “actual” baseline response for each individual subject may be, making determination of the change in response difficult to measure. This is not an issue in animal research in which drug exposure can be completely controlled.
Sensitization has also been studied in individuals with drug use disorders. In long-term cocaine abusers, sensitized subjective effects or physiological responses were reported to be absent (e.g., 156); however, without initial response data, the accuracy of this conclusion cannot be evaluated. Even after a drug-free period, the response of these individuals to the initial drug exposure in these studies may have been sensitized from the history of drug exposure, resulting in no change in response upon drug re-exposure (i.e., a ceiling effect). Also, it is common for these studies to use only two drug exposures to assess the presence or absence of sensitization, which may have been inadequate for demonstration of this phenomenon. Other important factors in human studies examining behavioral sensitization are dose, dosing interval, and time elapsed between the cessation of treatment and test. Animal research shows that for classical psychostimulant drugs like cocaine and amphetamines, intermittent drug administrations of moderate or high doses are optimal for achieving drug-induced behavioral sensitization (5, 127, 170). Similarly, studies in humans using amphetamine have shown that repeated administrations of higher doses (20–30 mg; p.o.) of this drug, but not lower doses (5–10 mg), are required to observe sensitized responses (143, 148, 150). Some animal research has also indicated that an “incubation” period after the cessation of drug treatment may be important for the establishment of long-term central nervous system changes that accompany sensitization (72, 171, 172). These factors are discussed in greater detail in Section 4.2. To the best of our knowledge, this factor has not been explored in studies involving human subjects.
1.5.2 Neural Sensitization
Behavioral sensitization in the form of stereotypic behaviors, as an apparent result of chronic pro-DAergic treatment, has been seen in Parkinson’s disease patients undergoing DA replacement therapies and described in relation to the similar abnormal involuntary movements (i.e., stereotypies) seen in methamphetamine abusers (173, 174). In fact, the mechanisms of behavioral sensitization during DA replacement therapy have been suggested to be very close to the neurobiology underlying the sensitizing effects of methamphetamine. This homology has been seen not only at a behavioral level, but also when several neurochemical measures are taken into account in human studies (i.e., DA receptor stimulation, changes in transduction pathways, gene expression, and alterations in the phenotype of striatal neurons) (see 173, 174).
Evidence for sensitization of amphetamine-induced striatal DA release has been described recently in humans. In 2006, Boileau and colleagues (143) used the radiolabeled tracer [11C]raclopride (a DA D2/D3 antagonist) in a positron emission tomography (PET) study that also included registration of anatomical magnetic resonance imaging (MRI). In this study, subjects without a history of drug abuse received three amphetamine (0.3 mg/kg) administrations, orally, given every other day with a withdrawal period of 2 weeks or 1 year. Then, a fourth amphetamine administration was given in the same context (the PET scan). A decrease in [11C]raclopride binding was interpreted as an increase in dopamine release. A greater decrease in binding was seen after the fourth administration as compared to the first, which was interpreted as sensitization to the DA-releasing effect of amphetamine. This effect was found at the level of the ventral striatum, which includes the nucleus accumbens, as well as the sensorimotor putamen. Corresponding with the neurochemical outcome was sensitization of behavioral variables, including ratings of alertness and energy (143).
A number of studies in humans have failed to find behavioral or neurochemical sensitization (some of these studies are also included in Table 11.1). In those in which sensitization was demonstrated, healthy subjects without a history of drug abuse were used, drug administration and tests were context-dependent, and moderate-to-high doses of psychostimulants were used. It is noteworthy that unlike pre-clinical research, to the best of our knowledge, there are no data available for drugs like ethanol or opiates showing neurochemical sensitization in humans; only cocaine and amphetamines have been studied.
1.5.3 The Ideal Human Study?
Because we do not perform research using human subjects, we are not qualified to provide a detailed description of a human study of sensitization, as we do for measurement of the same in mice (see Section 3. However, we will take this opportunity to express our thoughts about what characteristics we believe to be important in the ideal human study. The first would be that individuals that are relatively drug naïve be used so that a measure of initial drug sensitivity can be obtained. Repeated drug exposure and response measurement in the same drug-associated context would also be part of the design. Another important characteristic would be that objective measures of sensitivity be used. Depending upon the drug, these could include heart rate, skin temperature, eye-blink rate, the use of actimeters for objective measurement of activity, and neural imaging. However, clearly an advantage to using human subjects over animal subjects is their ability to express how they are feeling, and descriptive questionnaires should also be part of the study design. In this case, it would be beneficial to consider questions or objective ratings that specifically target vigor and energy apart from general mood. Also important would be examination of the data for individual variability in initial drug response and change in response. As has been seen in animals, some individuals may be more susceptible to the development of sensitization than others and this susceptibility may or may not be associated with their initial sensitivity. It is important to take this into consideration when drawing conclusions about whether humans did or did not display sensitization in a particular study. Finally, because animal studies have suggested that an incubation period may reveal unique information about drug-induced sensitization, the ideal human study would also include a drug wash-out period followed by a final drug challenge. All of this said, we recognize that because drug-induced sensitization has been shown to be long-lasting and to influence drug self-administration, there are certain risks in performing the ideal study in humans that may be impossible to overcome.
2 Materials
The methods for measuring drug-induced sensitization in animals and humans are quite different and require different materials. In addition, methods for measuring behavioral versus neurochemical sensitization require completely different analytical tools. We will describe herein only the materials and methods relevant to psychomotor sensitization measurement in mice, using injected drugs.
(1)
Calibrated 1-ml injection syringes with 0.4-mm, 27-ga hypodermic needles
(2)
Vehicle for injection
(3)
Drug in the appropriate concentration(s) for injection
(4)
Automated activity monitoring equipment – we use 40 × 40 × 30 cm AccuScan monitors (AccuScan Instruments, Columbus, OH)
(5)
Ventilated housing chambers for the monitoring equipment to exclude external light and noise
(6)
A scale for measuring body weight
(7)
Bedding-lined holding cages
3 Methods
(1)
On each day, move mice in their home cages to the testing room about 1 h prior to injection or testing and leave them undisturbed to allow them to acclimate to the test room.
(2)
On each test day, prepare one bedding-lined holding cage for each locomotor chamber to be used. Weigh each mouse to be tested in the first test pass and place each in a separate holding cage within 10 min of testing.
(3)
On test days 1 and 2, inject each mouse intraperitoneally (i.p.) with vehicle (saline) immediately prior to placement in the activity chamber and test for 5–20 (ethanol or other rapid, short-acting drugs) or 15–60 (methamphetamine or other drugs with longer-duration effects) min; the duration of test can be as long as desired, but data should be collected in relatively short-time intervals (1–5 min) so that the time course can be examined. Data collected on day 1 will provide baseline activity data in a novel environment. Data collected on day 2 will provide baseline activity data in a familiar environment.
(4)
Return mice to their home cages after testing on each day and return mice to colony room after all mice have been tested.
(5)
Examine day 2 baseline activity data to be certain that groups to be treated differently on subsequent days are well matched for activity level. We recommend that if photocell beam interruptions are used to provide the measure of activity, then a monitoring system that can translate these data into distance traveled be chosen. Arrange mice into treatment groups so that they are matched for baseline activity level, also taking into consideration the important factors in your experiment, such as litter, strain, and sex.
(6)
On test day 3, inject one group of mice i.p. with vehicle (Vehicle Control) and the other group i.p. with drug (Drug Group; this is for a single-dose study). Begin testing immediately after injection as on days 1 and 2. Data collected on day 3 will provide a measure of acute stimulation, when compared to the day 2 baseline.
(7)
Treatment on subsequent days will depend upon the drug to be tested:
For methamphetamine and cocaine (see Table 11.2)
(a)
On days 4, 6, 8, and 10, leave animals undisturbed in their colony room.
(b)
On days 5, 7, and 9, inject with vehicle or the same treatment dose as on day 3, and test as on day 3.
(c)
On day 11, inject all mice with drug and test. Day 11 data will provide a measure of sensitization by comparing the repeated Vehicle Control group with the repeated Drug Group. In addition, the increase in drug response on day 11 for the repeated Drug Group, compared to their response on day 3 will provide a within-group measure of sensitization. A blood sample can be obtained on this day after testing to measure blood drug concentration in mice receiving drug for the first time versus those that have received drug repeatedly.
(d)
Finally, on day 12, inject all mice with vehicle and test. Day 12 data will provide a measure of conditioned activation – mice that have received drug repeatedly paired with the test chamber may exhibit higher levels of activity on this day than those that have received vehicle on most days in the same environment.
(e)
If desired, wait for a period of 1–3 weeks and challenge the repeated Drug Group mice with drug to examine whether sensitization continues to be expressed.
Table 11.2
Summary of test protocol for cocaine- or methamphetamine-induced locomotor sensitization
Day: | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
Vehicle Control | Veh | Veh | Veh | None | Veh | None | Veh | None | Veh | None | Drug | Veh |
Drug Group | Veh | Veh | Drug | None | Drug | None | Drug | None | Drug | None | Drug | Veh |
Test | Test | Test | – | Test | – | Test | – | Test | – | Test | Test |
Figure 11.1 gives an example of data for a cocaine sensitization study through step (d). Mice in this experiment were adult, male and female mice from a line selectively bred for low levels of voluntary methamphetamine consumption. The dose of cocaine was 10 mg/kg. They were tested for 15 min, with data collected in 5-min time intervals. Note (1) the similarity of the two groups in locomotor activity after saline on days 1 and 2, (2) the acute response to cocaine of the Cocaine Group on day 3, (3) the similarity of the acute cocaine response of the Vehicle Control on day 11 to that of the Cocaine Group on day 3, (4) the gradual increase in cocaine stimulant response of the Cocaine Group from day 3 to day 11 (this is the measure of within-group sensitization), (5) the difference in response to cocaine between the two groups on day 11 (this is the measure of between-groups sensitization), and finally (6) the slightly increased locomotor behavior after saline on day 12 of the Cocaine Group compared to the Vehicle Control (not quite significant in this case), suggesting some conditioned activation associated with repeated treatment with cocaine in the test environment.
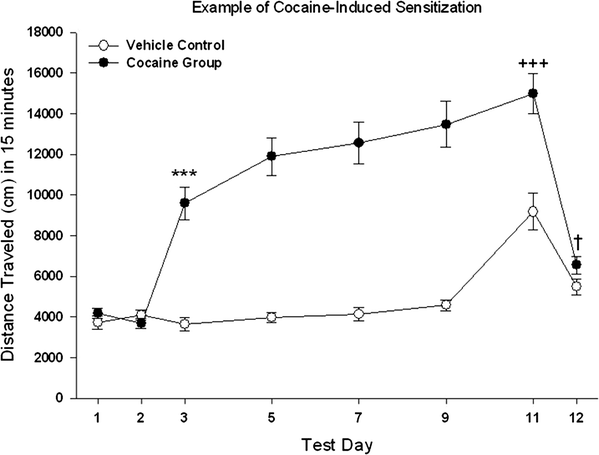

Fig. 11.1.
Mean ± SEM for distance traveled during 15-min tests following the protocol described in Table 11.2. Vehicle Control mice received 0.9% saline on all days shown except day 11, when they received an i.p. injection of 10 mg/kg cocaine HCl. The Cocaine Group received 0.9% saline on days 1, 2, and 12, and 10 mg/kg cocaine on all other days. Mice were left undisturbed on days 4, 6, 8, and 10. *** p < 0.001 for the comparison of the two groups on day 3. +++ p < 0.001 for the comparison of day 3 to day 11 within the Cocaine Group and for the comparison of the two groups on day 11. † p = 0.07 for a statistical trend toward a difference between the two groups on day 12.
For ethanol (see Table 11.3)
(a)
On days 4, 5, 7, 8, 10, and 11, weigh mice, treat with saline or ethanol (in colony room or in test room), and return them to their home cages – holding cages are not used on these days.
(b)
On days 6 and 9, weigh mice and place in holding cages, inject with saline or ethanol, and test as on day 3.
(c)
On day 12, inject all mice with ethanol and test. Day 12 data will provide measures of between-group and within-group sensitization. A blood sample can be obtained on this day after testing to measure blood ethanol concentration in mice receiving ethanol for the first time versus those that have received ethanol repeatedly.
(d)
On day 13, inject all mice with vehicle and test. Day 13 data will provide a measure of conditioned activation.
(e)
If desired, wait for a period of 1–3 weeks and challenge the repeated Drug Group mice with drug to examine whether sensitization continues to be expressed.
Table 11.3
Summary of test protocol for ethanol-induced locomotor sensitization
Day: | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
Vehicle Control | Veh | Veh | Veh | Veh | Veh | Veh | Veh | Veh | Veh | Veh | Veh | EtOH | Veh |
Drug Group | Veh | Veh | EtOH | EtOH | EtOH | EtOH | EtOH | EtOH | EtOH | EtOH | EtOH | EtOH | Veh |
Test | Test | Test | – | – | Test | – | – | Test | – | – | Test | Test |
Figure 11.2 gives an example of data from an ethanol sensitization study through step (d). Mice in this experiment were adult, male DBA/2 J strain mice and the dose of ethanol used on treatment and test days was 2 g/kg. Mice were tested for 20 min, with data collected in 5-min time intervals. Note (1) the similarity of the two groups in locomotor activity after saline on days 1 and 2, (2) the acute response to ethanol of the Ethanol Group on day 3, (3) the similarity of the acute ethanol response of the Vehicle Control on day 12 to that of the Ethanol Group on day 3, (4) the increase in ethanol stimulant response of the Ethanol Group from day 3 to day 12 (this is the measure of within-group sensitization), (5) the difference in response to ethanol between the two groups on day 12 (this is the measure of between-groups sensitization), and finally (6) the similarity of the two groups in locomotor behavior after saline on day 13, indicating no conditioned activation in the Ethanol Group in this study.
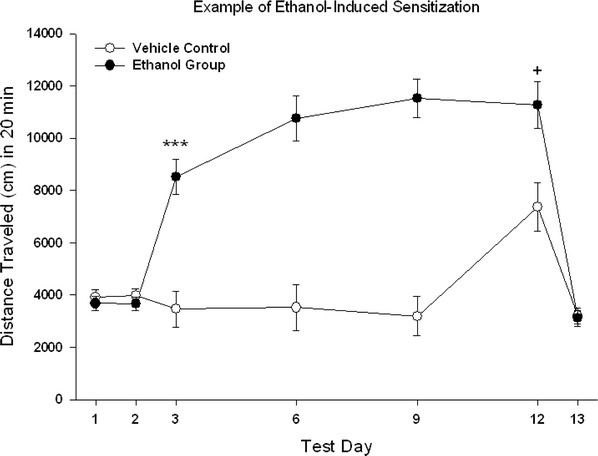

Fig. 11.2.
Mean ± SEM for the distance traveled during 20-min tests following the protocol described in Table 11.3. Vehicle Control mice received 0.9% saline on all days shown except day 12, when they received an i.p. injection of 2 g/kg ethanol. They received saline injections in their colony room and were then returned to their home cages on intervening days 4, 5, 7, 8, 10, and 11. The Ethanol Group received 0.9% saline on days 1, 2, and 13, and 2 g/kg ethanol on all other days, including the intervening days between test days, when they were treated in the home cage. *** p < 0.001 for the comparison of the two groups on day 3. + p < 0.05 for the comparison of day 3 to day 11 within the Ethanol Group and for the comparison of the two groups on day 11.
4 Notes
4.1 Treatment Issues
There is an array of factors to consider when designing an experiment to measure susceptibility to drug-induced sensitization or to identify underlying mechanisms. Because it may be desirable to examine both enhancement and attenuation of sensitization, it may be useful to have methods that produce submaximal as well as maximal levels.
Factors to consider are dose, number of treatments, frequency or interval between treatments and challenge, and duration of testing. It was shown almost three decades ago that more intermittent treatment schedules produce a greater magnitude of locomotor sensitization to methamphetamine, but that the effect of interval between treatments was dependent upon dose (127); more robust sensitization was seen with longer intervals between treatments for higher methamphetamine doses. In a study that extended examination of treatment interval to other drugs of abuse, a treatment interval of 24 h or longer was needed to induce locomotor sensitization to methamphetamine, cocaine, and morphine (170). The same treatment schedule may produce different behavioral responses, even for drugs with similar mechanisms of action, like cocaine and methamphetamine (e.g., 175). These factors create some lack of clarity with regard to choosing a dose and treatment interval. However, for classical stimulant drugs like cocaine and amphetamines, it seems to be advisable for the induction of sensitization to utilize an intermittent treatment schedule in which drug is allowed to fully clear between treatments, rather than a more chronic treatment schedule (176).
For ethanol, we have found more reliable and robust sensitization with a 24-h treatment interval; however, both daily (38, 59, 74, 177) and less frequent (56, 178) treatments have been shown to induce sensitization to ethanol. A systematic study showed that in Swiss mice, treatment intervals of 24, 48, and 96 h induced similar degrees of ethanol-induced sensitization (179). However, it is currently unknown whether there are genotype-dependent effects of treatment interval, because this has not been systematically studied. On the other hand, dose of ethanol has been clearly shown to play an important role in both the development and expression of sensitization (179, 180). In our experience, sensitization to ethanol can be reliably induced using a 24-h treatment interval, with ethanol doses of 1.5 g/kg or greater, in strains that are susceptible to ethanol-induced sensitization. Higher doses for the induction of sensitization are advisable in less sensitive genotypes (e.g., 180). Sensitization can be induced by three or fewer exposures to ethanol in some mouse strains, but may require a larger number of exposures in others (181–183). Also, for ethanol, we have found that at least the sensitization-resistant C57BL/6 J strain is more likely to show sensitization using a repeated injection procedure that does not include test environment exposure during the treatment phase (182).
Pharmacokinetic/pharmacodynamic factors likely provide a partial explanation for the influence of treatment interval on magnitude of sensitization. Longer treatment intervals may be required for drugs like methamphetamine that have a longer half-life if a drug-free period between administrations is important in the sensitization process. In fact, at least for ethanol, sensitization has been described as a kindling-like process that could influence craving (184) and may require recurrent cycles of exposure, drug clearance, and withdrawal to fully develop (185). Repeated cycles of chronic ethanol exposure with intervening drug-free periods have been shown to result in increased ethanol intake compared to levels seen after a single episode of chronic exposure (186, 187). An escalating dose, binge-like model of cocaine administration has been advocated for studying sensitization, as an exposure model that might better reflect escalating drug use (188). This may be a good choice, depending upon the goal of the drug administration model. In a study of non-treatment-seeking cocaine users, the effects of escalating doses of smoked cocaine were studied. Sensitization of heart rate, blood pressure, positive drug effect ratings, and cocaine liking were found in the escalating dose group, but not in the fixed dose group (147).
4.2 Test Issues
Test frequency and duration should also be considered when designing a sensitization study. Tests after every drug administration or every few administrations allow the acquisition pattern to be tracked. However, acquisition pattern may vary from experiment to experiment even when identical procedures are used with the same type of mouse in the same laboratory. For example, results for two groups of CFW mice tested for the acquisition of ethanol-induced sensitization were presented in a single paper (189). In both groups, robust sensitization was demonstrated. However, in one group, maximum sensitization was present on the second test day, which was after the fourth ethanol treatment, whereas in the other group, a progressive increase in sensitization was evident across two additional tests, after the seventh and tenth ethanol treatments. One possible explanation for the differences in this case is individual differences in susceptibility to sensitization. CFW mice are a genetically heterogeneous stock and there could be genotype-dependent differences among individual animals that would be revealed in mean response differences. Another possible source of variation is environmental. Although the data for the two experiments were collected in a single laboratory, using common equipment and methods, it is not known if the same person collected both data sets or during what season of the year the data were collected and what impact such variables may have had. Others have shown that one cannot completely control environmental factors that may influence experimental results (190, 191), even when using genetically identical individuals (i.e., inbred strains).
Duration of the behavioral test should take into account the pharmacodynamics of the drug which affect the duration of the behavioral response. For example, for ethanol the behavioral stimulant effects are rapid and relatively short-lived (e.g., 87, 192, 193), so a shorter test duration (∼15 min) may be appropriate. For methamphetamine, the stimulant effects occur rapidly after administration, but last longer (194, 195); thus, a somewhat longer test duration (∼60 min) may be desirable if the goal is to measure behavior for the entire duration of the drug response; however, sensitization can clearly be detected during earlier time points for drugs with longer durations of action (e.g., 2, 196, 197), so shorter test periods may also suit the goals of the research. Clearly, time is an important factor to include in the analysis of sensitization data. If data are accumulated into a single, long test period, but effects occur and subside rapidly, these transient effects may be difficult to detect without consideration of time-dependent patterns of response.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


