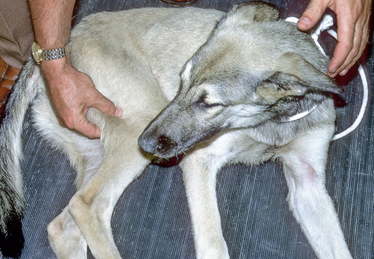
Chapter 90 Bacterial meningitis involves inflammation of the subdural covering of the brain and spinal cord, including the arachnoid membrane, subarachnoid space, pia mater, and cerebrospinal fluid (CSF), in response to a bacterial infection. The subarachnoid space projects into the nervous tissue alongside penetrating blood vessels. Bacterial infections can spread into the meningeal spaces from natural orifices such as the nasal sinuses, retrobulbar tissues,1–3 and middle or inner ear.4 Bacteria that proliferate in middle or inner ear infections can enter the cranial vault and produce cranial meningitis or subdural abscess formation (empyema) and, frequently, central vestibular dysfunction.5–8 Bacteria can also enter the meningeal spaces through traumatic focal penetration (foreign bodies or bite wounds),9,10 spread from sites of infection in adjacent venous sinuses or bony structures, or spread via hematogenous arterial dissemination.11,12 Vascular spread can result in lodgment of bacteria in the endothelial cells of capillaries or the choroid plexus, which results in bacterial invasion of the parenchyma of the central nervous system (CNS) or colonization of the CSF, respectively. In the CSF, bacteria have adequate nutrients and a paucity of phagocytes and immunoglobulin, and they can proliferate, which produces a progressive inflammatory process that can readily spread along CSF pathways. Bacteria that cause meningitis in dogs or cats are varied and can include aerobic, microaerophilic, and anaerobic species. Staphylococci, streptococci, Pasteurella spp., Actinomyces spp., Nocardia spp., and a variety of anaerobic bacteria have been isolated.5,13–15 On entry into the CSF, bacteria can replicate to a moderate degree before they trigger a host protective response, because the immune response in this area is slow. The immune response of the host, rather than virulence of the pathogen, is usually responsible for most of the subsequent pathologic events. Toll-like receptors (TLRs), which are present on astrocytes, neurons, microglia, and oligodendrocytes, initiate innate recognition of the microorganisms. TLR signals initiate the release of a wide variety of proinflammatory mediators including reactive intermediates, cytokines, and chemokines within the CNS tissues and CSF spaces.16 Within the nervous tissue, inflammatory processes lead to increased expression of TNF-α; thus, higher CSF concentrations of TNF-α have been used to distinguish bacterial from aseptic meningitis in humans.17,18 Immunologic damage to bacteria leads to the release of breakdown products into the CSF and local CNS tissue. This further stimulates release of cytokines and prostaglandins, which are chemotactic for leukocytes and contribute to meningeal and ependymal inflammation. Subsequent inflammation leads to vascular injury and vascular thrombosis; tissue inflammation, edema, and necrosis; and increased CNS pressure within the confines of the dura mater and bony spaces. Inflammatory processes may also lead to adhesions and obstruction to CSF flow, resulting in obstructive internal or external hydrocephalus.19 Elevated rectal temperature may be found on examination because of fever, as well as excessive muscular exertional activity. Animals walk with a short stiff stride. Often the back of affected dogs is arched and the neck is outstretched with the head pointing in a downward direction. Paraspinal and extremity hyperesthesia is present with limb and spinal manipulation (Figure 90-1). When inflammation is confined to the meninges, postural reactions that involve motor responses are often stilted. As the inflammation progresses into the CNS parenchyma, deficits in sensory and motor responses are observed. Delayed placing reactions, paresis, and ataxia can be observed if cerebrospinal pathways are affected. Cranial nerve deficits are often observed on neurologic examination. Further signs of cerebral dysfunction include signs such as altered mentation (depression, aggression) or seizures. Myotatic and flexor reflexes may be difficult to elicit because of increased muscle tone from the animal’s extreme tenderness and unwillingness to relax. Loss of reflex function occurs if spinal nerves become damaged in the inflammatory process. Inflammation or compression of muscle, bone, and joints can be initially confused with that of nerve root or peripheral nerve inflammation. The differential diagnosis for bacterial meningitis includes other infectious causes of meningeal irritation including that caused by viral, rickettsial, fungal, protozoal, and algal organisms (Table 90-1).20 Feline infectious peritonitis is probably the most common cause of meningomyelitis in cats.21,22 When parenchymal neurologic dysfunction is apparent, such as evidenced by delayed placing reactions, then protozoal meningoencephalomyelitis should also be considered. Frequently, more localized hyperesthesia associated with upper or lower motor neuron deficits can be observed with epidural empyema, discospondylitis, or paraspinal osteomyelitis. Steroid-responsive meningitis-arteritis in dogs can mimic all the signs of bacterial meningitis.23 Diseases associated with myositis (such as American canine hepatozoonosis and leptospirosis) can also produce clinical signs that resemble those of meningitis. TABLE 90-1 Examples of Infectious Agents That Can Cause Meningitis in Dogs and Cats∗ ∗Many of the listed agents also cause encephalitis or encephalomyelitis in association with meningitis. †Via dissemination or cribriform plate destruction as a result of extension of nasal cavity disease. Although there are no hematologic or biochemical abnormalities that are specific for bacterial meningitis, the CBC may reveal leukocytosis, often with a left shift, especially if the process that leads to the meningitis resulted from hematogenous spread; however, an absence of neutrophilic leukocytosis is common in dogs or cats with meningitis.15 Serum chemistry abnormalities are variable and nonspecific and can include increased ALT and ALP activities, hyperglycemia, and variable changes in serum electrolytes. Definitive diagnosis of bacterial meningitis requires evaluation of the CSF. Precautions should be taken not to remove too much fluid if CSF pressures could be increased. If imaging capabilities are available, they should be performed and evaluated before CSF removal, since they may reveal evidence of increased intracranial pressure. However, findings on medical imaging should not be used to eliminate CNS inflammatory disease, because CSF findings have the highest sensitivity for its detection.24 Abnormalities found on CSF analysis in animals with bacterial meningitis usually consist of marked neutrophilic pleocytosis (Figure 90-2), and CSF protein concentration is increased. CSF protein concentration (often >100 mg/dL) and leukocyte numbers (usually >100 cells/µL) tend to be greatest in suppurative meningitis (especially of infectious origin) as compared to other CNS diseases; however, the electrophoretic patterns of the proteins are not specific for a diagnosis.25 Neutrophils generally predominate; however, especially in chronicity or with concurrent antimicrobial use, monocytes, lymphocytes, or eosinophils may be observed. In human bacterial meningitis, glucose levels in the CSF are reduced because of inflammatory cell utilization, and lactic acid levels may be increased. Further studies are needed to determine if these can be used in dogs and cats to differentiate bacterial from other causes of meningeal inflammation. Finding organisms in the CSF on cytologic examination is exceedingly rare, and isolation of organisms in culture is also uncommon despite their visible presence on histopathologic examination. FIGURE 90-2 Cytologic appearance of CSF (cytospin preparation) in a 3-year-old male neutered Labrador retriever with bacterial meningitis.
Bacterial Meningitis
Etiology and Epidemiology
Clinical Features
Physical Examination Findings
Diagnosis
Dogs
Cats
Viruses
Canine distemper virus
Arboviruses
Suid herpesvirus 1 (pseudorabies)
Feline infectious peritonitis virus
Borna disease virus
Suid herpesvirus 1 (pseudorabies)
Bacteria
Staphylococcus spp.
Streptococcus spp.
Pasteurella multocida
Enterobacteriaceae (including Salmonella spp.)
Actinomyces spp.
Nocardia spp.
Mycoplasma spp.
Anaerobes
Brucella canis
Bartonella spp.?
Borrelia burgdorferi?
Leptospira spp.?
Anaplasma phagocytophilum?
Rickettsia rickettsii
Neorickettsia helminthoeca
Ehrlichia canis
Streptococcus spp.
Pasteurella multocida
Nocardia spp.
Mycoplasma spp.
Anaerobes
Fungi
Blastomyces dermatitidis†
Coccidioides spp.
Cryptococcus spp.†
Aspergillus spp.†
Candida spp.
Histoplasma spp.
Phaeohyphomycoses
Hyalohyphomycoses (e.g., Paecilomyces spp.)
Cryptococcus spp.†
Protozoa
Toxoplasma gondii
Neospora caninum
Leishmania spp.
Balamuthia mandrillaris
Acanthamoeba spp.
Toxoplasma gondii
Algae
Prototheca spp.
Laboratory Abnormalities
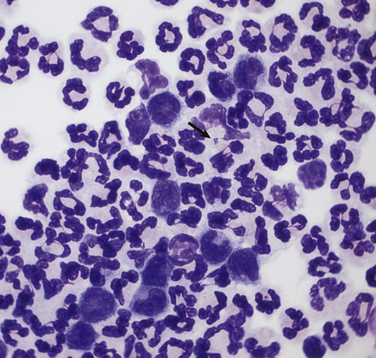
There is a large population of nondegenerate to moderately degenerate neutrophils. Many of the neutrophils contained intracellular cocci to pleomorphic short rod-shaped bacteria found in pairs and small aggregates (arrow). Moderate numbers of foamy macrophages and reactive lymphocytes are also present. CSF analysis revealed a total protein of 402 mg/dL (reference range, <25 mg/dL), 100 red blood cells/µL, and 5200 white cells/µL, 92% of which were neutrophils.![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Bacterial Meningitis
Only gold members can continue reading. Log In or Register to continue