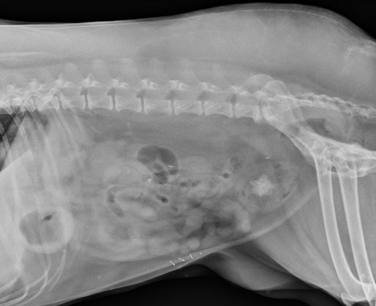
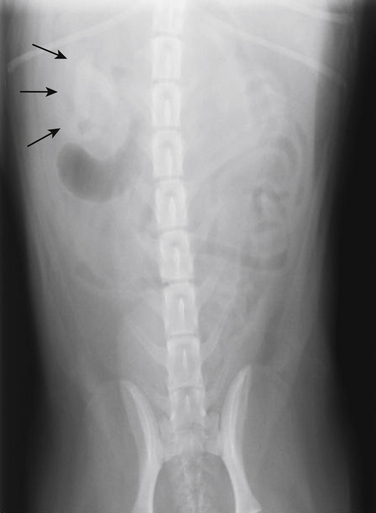
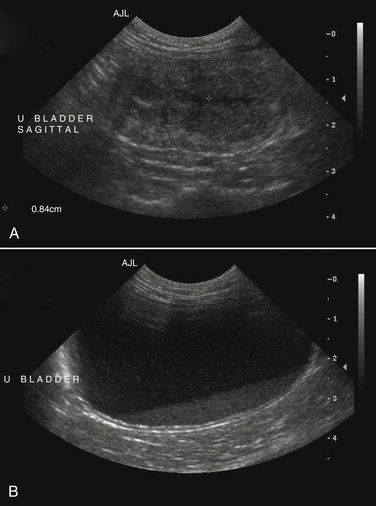
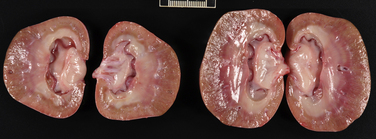
Chapter 89 In healthy dogs and cats, bacteria are not found in the upper urinary tract, urinary bladder, proximal urethra, and prostate gland. Factors that prevent bacterial colonization of the bladder include the glycosaminoglycan layer that covers bladder transitional epithelium, frequent voiding, the normal urethral microflora, antimicrobial properties of urine, an appropriate host immune response, and a functional urethral sphincter. Commensal bacteria that reside in the distal urethra, prepuce, and vagina invade opportunistically when these mechanisms are disturbed. The most common pathogen involved in all genitourinary tract infections is Escherichia coli, which has a particular propensity to adhere to epithelial cells of the genitourinary tract. Others include coagulase-positive and coagulase-negative staphylococci; Enterococcus spp.; other aerobic gram-negative bacilli such as Klebsiella, Proteus, Enterobacter, and Pseudomonas aeruginosa; Corynebacterium spp.; streptococci; and mycoplasmas. Anaerobic bacteria can also be found in the genital tracts of female dogs and can contribute to pyometra and metritis when host defenses are impaired. In addition to impaired host defenses, a variety of bacterial virulence factors influence the ability of certain bacteria to ultimately invade tissues and cause disease. Virulence factors of uropathogenic E. coli are described in more detail in Chapter 36. Bacterial UTIs are commonly diagnosed in dogs. Although mixed bacterial infections can occur, the vast majority of UTIs involve a single bacterial species.1 The most common uropathogen isolated is E. coli, which accounts for approximately 50% of all isolates, followed by Staphylococcus, Proteus, Klebsiella, Enterococcus, and Streptococcus species. Mycoplasmas have also been isolated from dogs with UTIs, although their clinical significance can be unclear because they are usually isolated from dogs that have other disorders of the lower urinary tract, such as underlying neoplasia or cystolithiasis. Spayed females and older dogs are at increased risk for bacterial UTIs; the mean age at diagnosis is 7 to 8 years of age.1,2 As a result of breakdown in host defense mechanisms, systemic disorders such as acute or chronic kidney disease, hyperadrenocorticism, diabetes mellitus, and systemic neoplasia also predispose dogs to UTIs (Table 89-1). TABLE 89-1 Factors That May Predispose (or Contribute) to Development of Urinary Tract Infections in Dogs and Cats In cats, bacterial UTIs are less common than in dogs. The prevalence of bacterial UTI in cats with lower urinary tract signs (i.e., stranguria, hematuria, pollakiuria, or dysuria; hereafter referred to as LUTS) evaluated by referral institutions has ranged from just 1% to 3%,3–5 although the prevalence was higher (23% of cystocentesis-collected specimens) in a study from Norway.6 Most young cats with LUTS have disorders such as feline interstitial cystitis, which are not associated with bacterial infection. When UTIs do occur in young adult cats, they are generally secondary to catheterization, perineal urethrostomy, or rarely, congenital anatomical defects. In older cats, UTIs often accompany diabetes mellitus, hyperthyroidism, and/or chronic kidney disease (CKD). The prevalence of UTIs in cats with diabetes mellitus is 11% to 13%.7,8 At two referral institutions, positive aerobic bacterial urine cultures were found in 17% and 22% of cats with CKD and 22% and 12% of cats with hyperthyroidism.7,9 Other factors that may predispose cats to UTI are breed (Persians or Abyssinians), female sex, older age, and lower body weight,5,8,9 although some of these risk factors may also be associated with the comorbidities mentioned earlier. As in dogs, E. coli is the most common infecting species in cats. Other potential uropathogens in cats include Streptococcus spp., Staphylococcus spp., Enterococcus spp., Klebsiella spp., Pasteurella spp., and Enterobacter spp.1,8,9 Mycoplasmas are very rarely isolated from the urine of cats.9 Uncommon forms of bacterial UTI in dogs and cats are encrusting cystitis and emphysematous cystitis. Encrusting cystitis is caused by the gram-positive bacterium Corynebacterium urealyticum. Urease production by this organism leads to precipitation of large amounts of struvite and calcium phosphate within the bladder and along the bladder and urethral mucosa, and in some cases within the ureters and renal pelvis as well.10–12 Most animals with C. urealyticum infections have severe LUTS. Emphysematous cystitis is characterized by the production of gas by bacteria within the urinary bladder wall (Figure 89-1). The most common cause is E. coli, but Clostridium spp. may also be involved.13,14 Most dogs and cats that develop E. coli emphysematous cystitis have glucosuria (usually secondary to diabetes mellitus), and gas production may result from fermentation of glucose to gas products by E. coli. In the absence of glucose, proteins such as albumin may be fermented to gas. Emphysematous cystitis caused by Clostridium perfringens occurs in the absence of diabetes mellitus.13,14 FIGURE 89-1 Lateral abdominal radiograph from a 10-year-old female spayed miniature schnauzer with emphysematous cystitis and a large, irregular cystic calculus. Irregular gas densities can see throughout the urinary bladder wall. Escherichia coli was cultured from a urine specimen that was obtained by cystocentesis. Definitions applied to infections of the urinary and genital tract are shown in Table 89-2. Simple uncomplicated UTI is a sporadic bacterial infection of the bladder in an otherwise apparently healthy individual with normal urinary tract anatomy and function.15 It has been estimated that uncomplicated UTI occurs in 14% of dogs that visit a veterinarian during their lifetime.16 However, the actual prevalence of simple uncomplicated UTI may actually be lower, because abnormalities in host defenses probably go unrecognized in many dogs. As noted earlier, uncomplicated UTI is rare in cats. Complicated UTIs are associated with concurrent disorders that predispose to recurrent or persistent UTI (see Table 89-1). TABLE 89-2 Definitions Applied to Urinary Tract Infections Recurrence of UTI may reflect refractory (or persistent) infection, relapsing infection, or re-infection (see Table 89-2). Without the use of molecular techniques (e.g., pulsed field gel electrophoresis), it is not possible to definitively distinguish between relapse of infection and re-infection if the same bacterial species is isolated repeatedly, because different strains may be present. Enterococcus spp. and Pseudomonas spp. are isolated more commonly from dogs with persistent or recurrent UTIs than from dogs with simple uncomplicated UTIs.2 E. coli can invade and form microcolonies within uroepithelial cells,17 which may contribute to persistent infection in the face of antimicrobial treatment. Bacteria may also be able to form biofilms in association with the bladder wall (“deep seated” infections), but studies that document this in the dog are lacking. Subclinical (or asymptomatic) bacteriuria is a term used in human medicine to describe the presence of bacteria in the urine as determined by a positive bacterial culture, in the absence of LUTS. This has generally been referred to as subclinical UTI in the veterinary literature. The use of the term subclinical bacteriuria in animals and its distinction from infection (which implies invasion of host tissues by bacteria and the associated inflammatory response) has been controversial, because subtle signs of infection (such as bladder pain, pollakiuria, or intermittent hematuria) may not always be detected. The term subclinical UTI could perhaps be applied to dogs and cats if cytologic evidence of infection (e.g., pyuria or hematuria) is present,15 but in human patients, the term subclinical bacteriuria is used even when pyuria is present. Subclinical bacteriuria or subclinical UTIs are commonly detected in dogs and cats, especially those with underlying endocrinopathies, renal failure, or neurologic disease, as well as dogs treated with glucocorticoids or cyclosporine.7,8,18–20 In a study of dogs treated with glucocorticoids for dermatologic disorders, 18% of 127 dogs had bacteriuria in the absence of clinical signs, whereas none of 94 dogs that had not received glucocorticoids had bacteriuria. Pyelonephritis most often occurs when bacteria ascend into the renal pelvis and parenchyma from the lower urinary tract. Less commonly, hematogenous spread to the kidney occurs. A single bacterial species (most often E. coli) is isolated from the urine of approximately three quarters of dogs with pyelonephritis; two species are isolated in fewer than 10% of dogs and three species from fewer than 5% of dogs. Pyelonephritis may be acute or chronic. Strains of E. coli that cause pyelonephritis have greater ability to adhere to cells than strains that cause cystitis. Adhesins located on the tip of E. coli fimbriae (P fimbriae) adhere to glycolipid residues on tubular, collecting duct, and bladder epithelial cells. In human pyelonephritis, both P and type I fimbriae are important in colonization of the renal pelvis.21,22 Other E. coli virulence factors associated with pyelonephritis include hemolysin, cytotoxic necrotizing factor, the iron scavenger aerobactin, and a serum protease known as Sat. Fimbriae are also important in the pathogenesis of Proteus pyelonephritis.23 The renal medulla is more sensitive to colonization than the cortex, possibly owing to impaired host defenses in a high osmolality, low pH, and low blood flow milieu. Organisms adhere to pelvic, distal, and proximal tubular epithelium and have been observed intracellularly. Considerable renal injury results from the inflammatory response to infection. Aggregation of neutrophils within capillaries and induction of vascular spasm by bacterial toxins and/or cytokines can contribute to renal ischemia. Diagnosis of UTIs is based on the clinical signs present, laboratory abnormalities, imaging findings, and culture of urine. The presence of fever or leukocytosis in addition to a positive bacterial urine culture should increase suspicion for concurrent bacterial pyelonephritis, prostatitis, or pyometra (see relevant sections later in this chapter). Ultrasound examination findings also can support the diagnosis and assist in the identification of underlying disorders such as neoplasia, anatomic abnormalities, or the presence of calculi or foreign material. At a minimum, collection of urine by cystocentesis followed by complete urinalysis (with sediment examination) and quantitative aerobic bacterial urine culture are recommended to properly confirm the presence of bacterial UTI for dogs or cats with signs of lower urinary tract disease.15 Additional diagnostics are indicated for animals that have recurrent LUTS or those with systemic signs such as fever, weight loss, or inappetence. The owners of animals diagnosed with what appears to be uncomplicated UTI should be advised that other underlying disease may be present. Dogs or cats with bacterial cystitis generally have no radiographic abnormalities. The urinary bladder may be small if pollakiuria is present. Emphysematous cystitis is characterized by the presence of radiolucencies within the bladder lumen and/or in association with the bladder wall (see Figure 89-1). Radiopaque cystic or renal (“staghorn”) calculi may be visible in dogs with struvite calculi secondary to Staphylococcus spp. or Proteus spp. infections (Figure 89-2). FIGURE 89-2 Dorsoventral radiograph of a 6-year-old female spayed shih tzu mix with a urinary tract infection caused by Proteus mirabilis and Enterococcus faecalis and nephrolithiasis. An opacity (“staghorn” calculus) occupies the right renal pelvis (arrows). Ultrasound examination of the urogenital tract provides information about the extent and severity of disease and underlying causes such as neoplasia, calculi, or foreign bodies. It does not permit visualization of the entire urethra, and so urethral abnormalities may be overlooked in some animals if only ultrasound is used to image the lower urinary tract. Ultrasound examination of the urinary bladder may be unremarkable or show a mild to moderately thickened bladder wall in dogs or cats with bacterial cystitis. Echogenicity of the urine may be present. Rarely, the bladder wall is severely thickened (Figure 89-3). Animals with emphysematous cystitis may have a hyperechoic urinary bladder wall with acoustic shadow artifacts. Shadow artifacts also occur when cystic calculi are present. FIGURE 89-3 Ultrasound images of the bladder of an 18-year-old female spayed domestic shorthair cat with diabetes mellitus, generalized osteoarthritis, a cervical myelopathy, and L4-L6 disc space compression and recurrent urinary tract infections. The cat had severe lower urinary tract signs (hematuria, pollakiuria, and stranguria), and a hemolytic Escherichia coli was isolated from the urine. A, The wall of the urinary bladder is markedly thickened. B, Resolution of bladder wall thickening after treatment with marbofloxacin for 3 weeks, which also resolved the clinical signs. Dogs or cats with pyelonephritis may have a normal abdominal ultrasound examination; or blunting of the renal papilla, pyelectasia, irregularity of the renal contour, and/or hydronephrosis may be seen (Figure 89-4). However, pyelectasia can occur in animals with normal renal function, diuresis, and outflow tract obstruction, and there is considerable overlap in the extent of dilatation between groups.24 The renal pelvis of dogs and cats with severe pyelonephritis may contain hyperechoic debris.25 Sometimes the surrounding mesentery is hyperechoic, and perinephric anechoic fluid is present as a result of localized peritonitis. Other nonspecific findings in pyelonephritis include increased renal cortical echogenicity and decreased corticomedullary definition. FIGURE 89-4 Kidneys of a 2-year-old male neutered German shepherd with diabetes mellitus and chronic pyelonephritis secondary to Klebsiella pneumoniae infection. The kidneys are bilaterally asymmetrical; the right kidney is larger than the left. There are several small, cortical subcapsular cysts, and the kidneys have an irregular conformation with markedly shrunken poles and are fibrotic. The renal parenchyma at the poles is markedly collapsed from the capsule to the pelvis, and the pelvis is uniformly dilated. (Image courtesy University of California, Davis, Veterinary Anatomic Pathology Service.) Cystoscopy can be a useful diagnostic procedure to identify underlying causes of recurrent UTIs in dogs and cats, after evaluation has been performed first to evaluate for metabolic abnormalities such as renal failure, hyperadrenocorticism, or diabetes mellitus. Cystoscopy or contrast computed tomography are the most sensitive diagnostic tests for identification of ectopic ureters. Cystoscopy can also be used to obtain mucosal uroepithelial biopsies for histopathology and culture. Quantitative aerobic bacterial culture and susceptibility is always indicated for dogs or cats with UTIs before starting antimicrobial drugs, because of the possibility of antimicrobial drug resistance, which is well recognized among E. coli strains isolated from dogs and cats with UTIs.26,27 Guidelines for diagnosis of bacterial UTIs in dogs and cats have been published.15 Ideally, urine should be collected by cystocentesis; if this is not possible, urethral catheterization can be used. Although any pathogens isolated from specimens collected by cystocentesis are likely significant, bacterial contamination from the skin is possible; therefore, the presence of more than 103 CFU/mL of bacteria is considered clinically significant. If specimens are collected via catheterization, colony counts that exceed 104 CFU/mL for male dogs and 105 CFU/mL for female dogs are considered clinically significant.15 Free-catch (midstream voiding) specimen collection should not be used for dogs or cats because it is highly susceptible to contamination. A positive aerobic bacterial culture of a cystocentesis-collected urine specimen confirms the presence of bacterial infection in animals that have LUTS. Culture can also help to differentiate re-infection from relapse should a UTI return; if the second isolate is a different bacterial species or has a different antibiotic susceptibility pattern (also known as an antibiogram), reinfection is likely.15 Specimens for culture should be refrigerated immediately and submitted to the laboratory as soon as possible. For specimens that reach the laboratory more than 24 hours after collection, culture results should be interpreted with caution, because false-negative and false-positive test results can occur. For dogs and cats with recurrent UTIs that undergo surgery, culture of a bladder wall biopsy or of cystoliths should be considered. Inexpensive urine “paddles” are available for in-house veterinary diagnosis of UTIs. One type consists of two different types of agar on either side of a plastic paddle (see also Chapter 3, Figure 3-6). The media are inoculated with urine collected by cystocentesis, and the paddles are then incubated and examined daily for growth. These paddles can be useful screening tools when client finances do not permit submission of specimens to a microbiology laboratory for culture, and they allow identification of significant bacterial growth by comparison of colony numbers to a chart provided by the manufacturer. They also reduce the effect of delays in submission of specimens to a laboratory. However, they may be inaccurate for identification of bacterial species involved, especially when mixed infections are present.28 Paddles may not yield reliable results if submitted to a laboratory for confirmation and minimum inhibitory concentration (MIC) testing. If used, and growth is detected on the paddle within 24 hours, saved (refrigerated) urine or a freshly collected urine specimen should be submitted to a microbiology laboratory for culture and susceptibility testing. Another type of in-house culture system provides limited information on bacterial antibiotic susceptibility (Flexicut Vet, Atlantic Diagnostics), but requires further study. In-house bacterial cultures should only be performed in clinics with appropriate laboratory facilities, proper biosafety level containment and waste management, and adequately trained personnel.15 Accurate susceptibility testing is difficult to perform and interpret, and whenever possible, submission to a veterinary diagnostic laboratory that adheres to Clinical and Laboratory Standards Institute (CLSI) guidelines is recommended. For animals with indwelling urinary catheters, some bacteriuria is expected due to compromise of host immune defenses. Culture is indicated if clinical signs of infection are present (e.g., gross hematuria, pyuria, or suspected bacteremia).15 Optimally, the catheter should be removed and cystocentesis performed after the bladder is allowed to fill with urine. Alternatively, the catheter could be removed, replaced with a new catheter, and a urine specimen collected through the catheter after several milliliters of urine are removed to clear the catheter. Urine culture should never be submitted from specimens collected from the urine bag, and there is no indication to culture the catheter tip. Laboratories that perform and report susceptibility testing for uropathogens should adhere to local regulatory guidelines (such as the CLSI). Breakpoints for systemic infections also apply to UTIs (see Chapter 3 for a discussion of breakpoints). However, many antimicrobials achieve much higher concentrations in the urine than they do in the serum. Therefore, if routine serum susceptibility testing is performed for a dog or cat with a lower UTI and normal renal function, some antimicrobials may eradicate the UTI, despite in vitro resistance. This is especially true for treatment of staphylococcal UTIs with penicillins and cephalosporines. Urinary breakpoints are available for ampicillin or amoxicillin in dogs (≤8 µg/mL), amoxicillin-clavulanate in dogs and cats (<8/4 µg/mL), and nitrofurantoin. Urinary breakpoints (reported as urine MICs by the laboratory) should be used only when infection is confined to the lower urinary tract. Treatment of UTIs consists of removal or management of the underlying cause(s) of infection, followed by antimicrobial drug treatment. Antimicrobial drug treatment alone without attention to an underlying cause frequently leads to recurrent infection and selection for resistant bacteria. Because of the increasing prevalence of multidrug-resistant (MDR) E. coli and staphylococci in dogs and cats with UTIs, consideration should be given to delaying antimicrobial drug treatment until the results of culture and susceptibility are available, provided the patient is stable and sepsis is absent. This is especially true for dogs and cats with recurrent UTIs that have a history of antimicrobial drug treatment. Empiric treatment while awaiting the results of culture and susceptibility could be considered to relieve discomfort, if present; however, some dogs respond well to analgesic therapy (e.g., tramadol) pending susceptibility test results. When sepsis is present, initial treatment should be broad-spectrum and cover MDR gram-negative bacteria (see Chapter 86). In most situations, rational choices for initial treatment of uncomplicated UTIs include amoxicillin or trimethoprim-sulfonamide (TMS) (Table 89-3), although TMS has the potential to cause significant adverse effects in dogs. Amoxicillin–clavulanic acid could be considered if practice/regional susceptibility trends suggest a high (>10%) prevalence of β-lactamase production by uropathogens isolated (i.e., >10% of bacteria isolated in a practice show susceptibility to amoxicillin–clavulanic acid but not amoxicillin).15 However, amoxicillin–clavulanic acid may need to be given q8h to ensure significant concentrations of clavulanic acid in the urine. Once culture and susceptibility results are available, the chosen antimicrobial drug should either be continued (if a clinical response has occurred) or switched to an alternative drug with activity against the pathogen that enters urine in high concentrations. A treatment duration of 7 days has been suggested.15 One study showed that treatment of dogs that had uncomplicated UTIs with high-dose enrofloxacin (20 mg/kg) for 3 days was not inferior to treatment with amoxicillin–clavulanic acid for 14 days.29 Additional studies of dogs with naturally occurring UTI that evaluate short durations of treatment with other antimicrobial drugs such as amoxicillin or TMS are needed. Studies are also needed to evaluate the need for posttreatment cultures to determine whether resolution of infection has occurred.15 TABLE 89-3 Suggested Initial Treatment for Stable Dogs or Cats with Genitourinary Tract Infections Pending Culture and Susceptibility Results See Table 86-8 for options for severe sepsis.
Bacterial Infections of the Genitourinary Tract
Lower and Upper Urinary Tract Infections
Term
Definition
Simple uncomplicated UTI
Sporadic bacterial infection of the urinary bladder in an otherwise apparently healthy individual with normal urinary tract anatomy and function
Complicated UTI
UTI that occurs in the presence of an anatomic or functional abnormality or comorbidity that predisposes to persistent UTI, recurrent infection, or treatment failure
Recurrent UTI
Three or more episodes of UTI during a 12-month period
Refractory UTI
Isolation of the same microorganism more than once in the face of treatment, despite in vitro susceptibility to the antimicrobial drug used
Relapsing UTI
Isolation of the same microorganism within 6 months of apparent clearance of the infection with treatment in between positive cultures
Re-infection
Isolation of a different microorganism within 6 months of apparent resolution of a previous infection
Subclinical bacteriuria
Presence of bacteria in the urine as determined by a positive bacterial culture, in the absence of LUTS; differentiation from subclinical UTI may be difficult
Diagnosis
Diagnostic Imaging
Sonographic Findings
Cystoscopy
Microbiologic Tests
Treatment and Prognosis
Uncomplicated UTIs
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Bacterial Infections of the Genitourinary Tract
Only gold members can continue reading. Log In or Register to continue
