Igor F. Canisso, Marco A. Coutinho Da Silva
Bacterial Endometritis
Endometritis is the main cause of subfertility and infertility in broodmares. It can be classified into infectious or noninfectious categories and can manifest clinically as an acute or chronic inflammatory process. During the past 30 years, controlled studies and clinical trials have advanced the understanding of endometritis pathophysiology and the natural mechanisms of uterine defense against infection. This allows distinction of uterine infections from the physiologic inflammatory response seen immediately after mating (i.e., breeding-induced endometritis) that results from uterine exposure to semen, extenders, and debris. After breeding, most mares are able to clear the infection or inflammation within 48 hours and are called “resistant” to endometritis; however, in about 10% to 15% of mares, the uterine inflammation persists, and these mares are called “susceptible” to endometritis. Often, susceptible mares have deficient defense mechanisms against infection (e.g., poor uterine contractility, anatomic abnormalities, and other conditions), which predisposes them to prolonged uterine inflammation and colonization of the uterus by microorganisms.
Until recently, bacterial endometritis was thought to cause only superficial infection of the endometrium and lumen. Moreover, in chronic cases of endometritis, the lack of response to treatment was usually ascribed to inappropriate treatment (from antimicrobial resistance or inappropriate frequency or duration of treatment) or reinfection secondary to poor management practices or failure to correct anatomic abnormalities. New insights on the pathogenesis of bacterial endometritis may explain some of the reasons for the chronicity of endometritis in some mares. By use of in situ hybridization techniques, investigators have identified deep streptococcal (Streptococcus equi subsp zooepidemicus) endometrial infection in mares. This microorganism apparently causes what have been termed dormant infections, and under certain undefined conditions, the infection becomes active. Identifying a method to reactivate the infection will facilitate proper diagnosis and treatment, but at present no such method has been established. To date, S equi subsp zooepidemicus is the only bacterial species identified as being capable of causing these dormant infections.
Another potential cause for establishment of chronic bacterial endometritis in the mare is production of biofilm by several microorganisms (e.g., Escherichia coli and Pseudomonas aeruginosa) commonly isolated from mares with endometritis. Although this biofilm has yet to be confirmed in the mare’s uterus, clinical trials to date have yielded positive results in treating chronically infected mares with mucolytics that can disrupt bacterial biofilm.
Etiology
Microorganisms causing bacterial endometritis can be classified as opportunistic pathogens and venereal pathogens. Endometritis caused by opportunistic infections is usually associated with deficient uterine defense mechanisms (e.g., poor lymphatic drainage and anatomic abnormalities), whereas venereal pathogens cause disease despite normal and efficient mechanisms of defense because of the high pathogenicity of the bacteria. Many opportunistic pathogens such as S equi subsp zooepidemicus and E coli are commensals of the caudal aspect of the reproductive tract and may be introduced into the uterus by multiple routes, including during breeding (both natural and artificial), during urogenital examinations, or as a result of breakdown of physical barriers to infection. The most common bacteria isolated from mares with uterine infections include S equi subsp zooepidemicus, E coli, Staphylococcus aureus, Klebsiella pneumoniae, P aeruginosa, and Bacteroides fragilis (Table 163-1). Taylorella equigenitalis, the causative agent of contagious equine metritis (CEM), is a true venereal pathogen in the mare. Taylorella equigenitalis infection is associated with profuse purulent vaginal discharge and pregnancy losses, and has the potential to significantly affect the horse-breeding industry. However, the pathogenicity of T equigenitalis during the last outbreaks in the United States in 2006 and 2009 appeared to have been reduced, compared with that seen during the initial outbreaks in 1977 in England and 1978 in Central Kentucky, with less than 1% of mares exposed to semen from contaminated stallions testing positive for the microorganism in the more recent episodes. It is worth mentioning that the initial outbreaks affected primarily Thoroughbreds (because of the requirement for natural covering), whereas the latest positive stallions were identified in breeds that allow artificial insemination. It is possible that the use of semen extenders containing antimicrobials limited the spread of infections.
TABLE 163-1
Aerobic and Anaerobic Bacteria Isolated From Mares With Endometritis
| Bacteria | Classification, Comments |
| Streptococcus equi subsp zooepidemicus | Opportunistic and potentially venereal, aerobic, gram positive; has been isolated in mixed infections with fungus; can cause chronic infections |
| Escherichia coli | Opportunistic, facultative anaerobic, gram negative; has potential to cause chronic infections, has been isolated in mixed infections with fungus |
| Pseudomonas aeruginosa | Potentially venereal, aerobic, gram negative; can be associated with chronic infections and mixed infections with fungus |
| Staphylococcus spp | Opportunistic, facultative anaerobic, gram positive, can be associated with chronic infections |
| Proteus spp | Opportunistic, anaerobic, gram negative, opportunistic bacteria |
| Enterobacter cloacae | Opportunistic, facultative anaerobic, gram negative |
| Klebsiella pneumoniae | Opportunistic and potentially venereal, facultative anaerobic, gram negative; capsule types 1, 2, and 5 can be venereally transmitted |
| α-Hemolytic streptococci | Opportunistic, aerobic, gram positive |
| Citrobacter spp | Opportunistic, aerobic, gram negative |
| Taylorella equigenitalis | Venereal, microaerophilic, gram negative; associated with severe purulent endometritis |
| Bacteroides fragilis | Opportunistic, anaerobic, gram negative; associated with mixed infections with aerobic bacteria |
| Fusobacterium mortiferum | Opportunistic, anaerobic, gram negative; associated with mixed infections with aerobic bacteria |
Because some stallions and mares may harbor venereally transmitted bacteria (e.g., T equigenitalis, K pneumoniae, and P aeruginosa), routine cultures of the stallion’s external genitalia and semen during the breeding season and of the mare’s genital tract before and after breeding are useful management tools to control disease spread, particularly when natural covering is performed.
Diagnosis
Diagnosis of endometritis is best performed by integrating information obtained from the mare’s reproductive history and clinical signs with findings from cytology, biopsy, and detection of bacteria in endometrial culture. Proper interpretation of findings from palpation and ultrasonography of the reproductive tract and from analysis of endometrial samples is necessary for successful diagnosis and treatment of the condition. Clinical signs of endometritis can be subtle or even absent, which makes diagnosis more challenging. A reproductive history of failure to conceive and short return to estrus despite good breeding management and use of a fertile stallion or semen can be suggestive of endometritis and should prompt further examination. Most mares with bacterial endometritis have intrauterine fluid accumulation, with or without vulvar discharge, and have poor perineal conformation that predisposes to pneumovagina, a pendulous uterus, or both. A speculum examination of the vestibule and vagina can be very informative and allows direct visualization of the cervix and vaginal mucosa, enabling detection of discharge through the cervix, urine pooling, pneumovagina, or other contaminants or problems in the caudal portion of the reproductive tract. Definitive diagnosis of bacterial endometritis can be achieved by evaluating endometrial cytology, culture, and biopsy specimens. Diagnosis and treatment of endometritis should be initiated at early estrus, when the cervix is open and the uterine defense mechanisms are maximal; alternatively, a dose of prostaglandin can be administered at the time of diagnosis to induce luteolysis and hasten return of estrus.
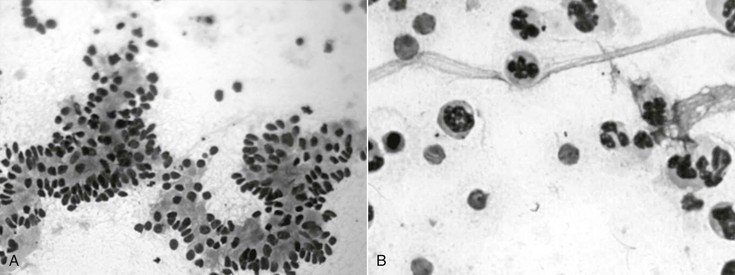
Specimens for endometrial cytology can be obtained with a double-guarded swab, double-guarded cytology brush, or low-volume uterine flush. Although all three methods provide for adequate sampling of the endometrium, use of a double-guarded cytology brush allows for preparation of smears of high cellularity and quality, with minimal background and no contamination from the vagina (Figure 163-1). Smears can be stained with Romanowsky’s or modified Wright-Giemsa stains. During evaluation at 1000× magnification, the smear is evaluated for elements of inflammation (i.e., frequent white blood cells, especially neutrophils) and for microorganisms (i.e., bacteria and fungi). Acute bacterial endometritis usually induces endometrial hypersecretion and subsequent intrauterine fluid accumulation, with pronounced neutrophilic exudate (Figure 163-2); on the other hand, chronic bacterial endometritis may induce more subtle signs, and sometimes there is no evidence of inflammation on endometrial cytology.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree