The towel technique is also better than gloves alone because the towel presents a larger surface area for the bird to try to evade. The bird is then less likely to try to bolt for freedom, whereas a bare hand is a much smaller target and encourages escape attempts.
For smaller cage birds, a piece of paper towel may be used and the bird transferred to a latex gloved hand. The neck of the bird should be held between the index and middle fingers. You can then use your thumb and forefinger to manipulate the legs or wings. The rest of the hand should gently cup the bird’s body to discourage struggling (Figure 11.2). It is still necessary to be cautious with this approach to prevent over-constraining the patient as this could cause physical harm.
Figure 11.2 Smaller Psittaciformes such as this budgerigar (Melopsittacus undulatus) may be restrained with one hand. Note the scaly beak infection.

In the case of particularly aggressive parrots, which are very difficult to handle, leather gauntlets may be used. Extreme care should be exercised as too strong a grasp around the bird’s body could prove fatal.
Other avian species
Toucans and hornbills
These have an extremely impressive beak, with a serrated edge to the upper bill. Providing the head is initially controlled using the towel technique previously described for parrots, an elastic band or tape may be fastened around the bill to prevent biting. The handler still needs to be careful of stabbing manoeuvres, and it is a good idea to work with a second handler who is solely responsible for containing the beak. Otherwise, restraint is the same as for the Passeriformes.
Columbiformes
These are restrained in a manner similar to the Psittaciformes (Figure 11.3). The following approach may be used, with the bird’s wings and feet cupped in one hand from the dorsum, while resting the sternum in the other hand.
Waterfowl
Restraint of these species is relatively straightforward but may become hazardous with the larger swan and goose family. The first priority is capturing the head. This can be done by grasping the waterfowl around the upper neck from behind. It is important to ensure that the handler’s fingers curl around the neck and under the bill, while the thumb supports the back of the neck and the potentially weak area of the atlanto-occipital joint. Failing this, a swan’s or shepherd’s crook, or other smooth metal, or a wooden pole with a hook attached, can be used to catch the neck – again high up under the bill. Care should be exercised with these ‘swan hooks’ as overzealous handling can lead to neck trauma.
Having restrained the head and beak, it is essential that you control the powerful wings by using a towel, thrown or draped over the patient’s back and loosely wrapped under the sternum. Many institutions have access to more specialised goose or swan cradle bags which wrap around the body, containing the wings but allowing the feet, head and neck to remain free.
The waterfowl may now be safely carried or restrained by tucking its body (contained within the towel or restraint bag) under one arm and holding this close to the torso. With the other hand, the handler may hold the neck loosely from behind, just below the beak.
Capturing escaped avian patients
Where a bird is loose, a number of methods can be used to capture it. Darkening the room and reducing its area, if possible, are both helpful to calm and confine the bird.
In the case of larger parrots, the use of a heavy bath towel thrown over the bird can confine it long enough to allow the handler to restrain the head from behind and then wrap the patient in the towel.
For very small birds, a fine aviary or butterfly net is extremely useful to catch the bird safely either in mid-flight or against the side of the cage or room. The mesh should be fine enough to ensure that no limbs or feathers will become entangled within it. The mesh is best made of a dark or black material to restrict light and calm the bird.
It is important to ensure that the patient is rapidly transferred from the net into the handler’s hands or a container as this is the most stressful period of the capture, and the inability to see whether the patient is in respiratory distress can lead to fatalities.
To recapture escaped raptors, a lure, baited with prey, can be used. Most raptors will remain within the area of their release for several days, and many captive-reared birds will not be able to kill prey for themselves. They will then become hungry and will often look for their handler to offer prey, either on the glove, if they are trained birds, or within the vicinity of their cage, if they are non-trained exhibit or breeding birds. Patience and persistence are two essential virtues when trying to recapture escaped raptors!
Aspects of chemical restraint
Assessment of the patient’s status
All methods of chemical restraint require that the patient is first restrained manually, even though it may be for a short period, while the medication is being administered. The aim is to keep the period of manual restraint to a minimum, in order to reduce stress.
Before any form of chemical immobilisation can be used, an assessment of the patient’s status must be made. The following points need to be considered:
- Is the procedure to be performed necessary for life-saving medication or treatment, or can it be postponed if the patient’s health is sub-optimal?
- Is the patient’s condition likely to be worsened by the anaesthetic drugs used?
Implications of avian respiratory anatomy and physiology
There are three main differences between avian and mammalian anatomy and physiology, which are important when considering restraint:
- It is difficult to suffocate the bird by constricting the neck.
- There is no ‘give’ to the trachea, and so inflatable cuffs on endotracheal tubes should not, if at all possible, be inflated as this will cause pressure necrosis in the lining of the trachea. In some cases, such as when flushing out the crop or proventriculus of the bird to remove foreign bodies, it may be necessary to inflate the cuff in order to prevent inhalation pneumonia. This must be performed with great care.
Pre-anaesthetic preparation
Blood testing
It may be advisable to run biochemistry and haematology tests on avian patients prior to administering anaesthetics, particularly in older and obviously unwell individuals. Blood may be taken from the following vessels:
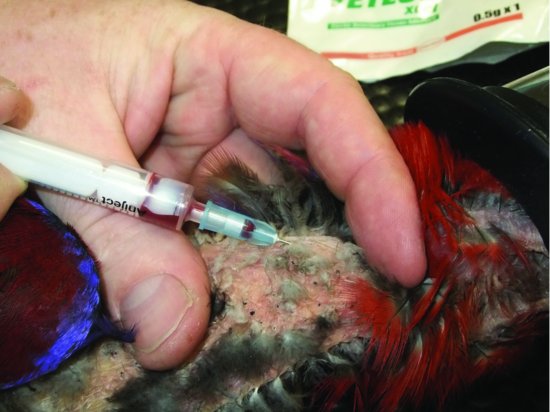
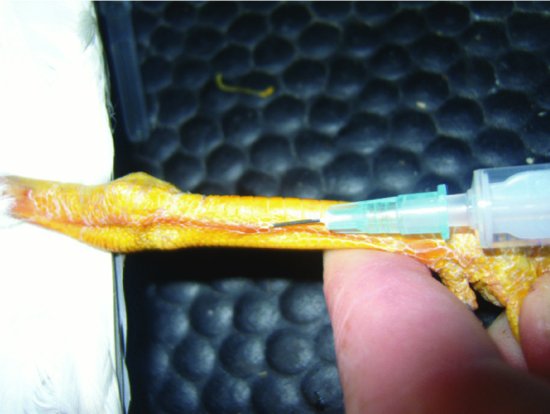
- The right jugular vein in nearly all species (it is significantly larger than the left) (see Figure 11.4).
- In the larger species the brachial vein, which runs cranially on the ventral aspect of the humerus (see Figure 11.5).
- In many waterfowl, long-legged birds and raptors, the medial metatarsal vein, which runs, as its name suggests, along the medial aspect of the metatarsal area (see Figure 11.6).
Fasting
Because of the high metabolic rate of avian patients, extended fasting may be detrimental to their health and their ability to recover from anaesthesia. This is because hepatic glycogen stores, which provide the most rapid form of stored energy, can be quickly depleted. Birds larger than 300 g bodyweight, which have larger glycogen stress, are slightly less likely to become hypoglycaemic with fasting.
The purpose of fasting is to ensure emptying of the crop. Fasting prevents passive reflux of fluid or food material during the anaesthetic which may then be inhaled, obstructing the airway or causing pneumonia.
Ideally, most birds are fasted between 1 and 3 hours depending on their body size. The smallest have the shortest period of fasting, often amounting to just 1–2 hours at most. Birds weighing over 300 g may be able to tolerate an overnight fast of 8–10 hours, assuming good health and body condition, and this may be necessary particularly if surgery on the gastrointestinal system or crop is intended. Whatever the period of fasting, water should only be withheld for 1 hour prior to anaesthesia.
Pre-anaesthetic medications
Pre-anaesthetic medications are infrequently used in birds. Of those used, fluid therapy is the most important. Whether it is crystalloid or colloid, pre-, intra- and post-operative fluids can make the difference between successful surgery and failure. Fluid therapy is covered in more detail in Chapter 14.
Antimuscarinic premedicants
Atropine and glycopyrrolate have been used as pre-anaesthetic medicants to reduce vagally induced bradycardia and oral secretions which may block endotracheal tubes. They both, however, may have unwanted side effects. The two main side effects are
- Causing unacceptably high heart rates, increasing myocardial oxygen demand and so increasing the risk of cardiac hypoxia and arrest.
- Making oral or respiratory secretions so thick and tenacious that they make endotracheal tube blockage even more likely.
Benzodiazepine premedicants
Diazepam or midazolam may be used in waterfowl as these species may exhibit periods of apnoea during mask induction of anaesthesia. This is a stress response (often referred to slightly inaccurately as a ‘diving response’) mediated by the trigeminal receptors in the beak and nares. When this response is triggered, the breath is held and the blood flow is preferentially diverted to the kidneys, heart and brain.
Induction of anaesthesia
Anaesthesia may be induced with two main categories of drugs: injectable anaesthetics and inhalational anaesthetics.
Injectable agents
Advantages of injectable anaesthetics include
- Ease of administration (most birds have a good pectoral muscle mass for intramuscular injections)
- Rapid induction
- Low cost
- Good availability
Disadvantages include
- Recovery is often dependent on organ metabolism.
- Reversal of medications in emergency situations is potentially difficult.
- Prolonged and sometimes traumatic recovery periods may ensue.
- Muscle necrosis at injection sites and lack of adequate muscle relaxation may occur with some medications.
The following are some of the injectable anaesthetics more commonly used in avian practice.
Ketamine and ketamine combination anaesthesia
When used alone, ketamine produces inadequate anaesthesia and recoveries are often traumatic, with the patient flapping wildly. Doses of 20–50 mg/kg are quoted (Forbes & Lawton, 1996). However, combining it with the benzodiazepines, diazepam (0.5–2 mg/kg) or midazolam (0.2 mg/kg) (Curro, 1998) helps with muscle relaxation and sedation, reduces flapping on recovery and allows the dose of ketamine to be reduced to around 10–20 mg/kg.
Ketamine may also be combined with xylazine (1–2.2 mg/kg) (Forbes & Lawton, 1996) or medetomidine (60–85 mg/kg) (Forbes & Lawton, 1996). This improves recovery, sedation and analgesia and again allows reduction of the ketamine dosage to 10–20 mg/kg depending on the species and procedure. The medetomidine may be reversed with atipamezole (the same volume as medetomidine is given). However, the alpha-2 drugs such as medetomidine have severe cardiopulmonary depressive effects and may compromise blood flow to the kidneys, risking renal damage. Sun-conures have been noted to be particularly intolerant of ketamine–alpha-2 combinations (Rosskopf et al., 1989).
The combined medications are usually given intramuscularly. Induction will take on average 5–10 minutes, but complete recovery may take 2–4 hours or more and is dose dependent.
It is also worth noting that ketamine is actively excreted from the proximal tubule of the kidneys, and so any kidney damage can lead to a prolonged recovery when using this drug.
Propofol
Propofol is given intravenously, preferably via a jugular, medial metatarsal or brachial vein catheter. It produces profound apnoea and is rarely used as an induction or anaesthetic agent in birds.
Inhalation agents
Nitrous oxide
This is rarely used in avian anaesthesia. It has good analgesic properties, but accumulates in large, hollow organs. There is some thought that it may therefore accumulate in the air sacs and may considerably prolong anaesthetic recovery times. Recent evidence disputes this, but it cannot be used on its own for anaesthesia. It must be used in combination with halothane or isoflurane to allow a surgical plane of anaesthesia to be reached.
Isoflurane
Isoflurane is the anaesthetic of choice for the avian patient. It is also licensed for use in avian species in the United Kingdom. Induction may be achieved by face mask at 4–5% concentration, reducing to 1.25–2% for maintenance, preferably via endotracheal tubing inserted into the trachea or an air sac. If using a mask for maintenance, an increase in gas concentration of 25–30% is required.
Advantages of isoflurane include
- Low blood solubility and minimal metabolism of the drug by the bird (<0.2%). This means that the drug does not accumulate in the bloodstream and is rapidly excreted by the patient, allowing rapid changes in anaesthetic depth.
- Minimal cardiopulmonary effects at sedative or light anaesthesia levels.
- Little or no tendency to cause cardiac arrhythmia.
- Unlike halothane, isoflurane does not require metabolism in the liver, making it suitable for sick avian cases.
- Cardiovascular arrest does not occur at the same time as respiratory arrest, allowing time for resuscitation.
- Isoflurane has a minimum alveolar concentration (MAC) (i.e. the concentration that produces no response in 50% of cases exposed to a noxious stimulus) of 1.44% in cockatoos (Curro et al., 1994).
Sevoflurane
Like isoflurane, it requires little or no organ metabolism and is very safe. Slightly higher percentages are required to induce and maintain the patient, but low blood solubility does allow rapid changes in anaesthesia levels (Greenacre, 1997). It also depresses plasma ionised calcium levels significantly, and so care should be taken when using in African Grey parrots which are prone to hypocalcaemia.
Maintenance of anaesthesia
For prolonged anaesthetic procedures, gaseous maintenance is required. Isoflurane and sevoflurane are currently the agents of choice.
For avian patients larger than 100 g, it is advisable to intubate the bird for more effective control of rate and depth of anaesthesia. Its respiratory depressive effects are also dose dependent. This means that with prolonged procedures, the patient may become apnoeic and require either manual or positive pressure ventilation. This can be more of a risk with some species, such as the African grey parrot, than with others.
Endotracheal intubation
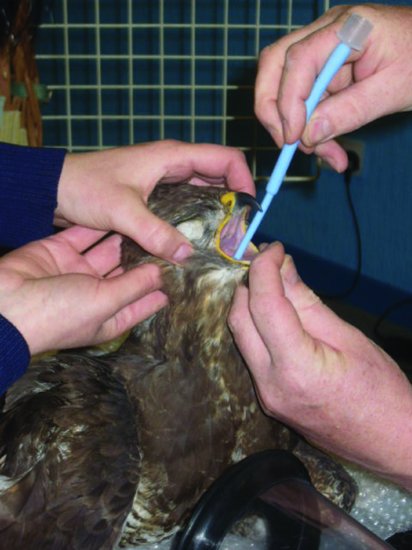
The beak must be held open by using an avian gag or by attaching short lengths of bandage to the upper and lower beaks. One handler can then use them to hold the beak open, while a second intubates the bird (see Figure 11.7). The glottis will be easily visible at the base of the tongue, in the midline. Intubation can be made easier if the tongue is grasped with atraumatic forceps, enabling it and the glottis to be pulled forward. Sizes of tube vary but would be typically a 3–3.5 F for a Grey parrot and up to a 4–5 F for a larger Macaw. The smaller tubes will block more easily with respiratory secretions, and the use of parasympatholytics does not stop this. Indications of tube blockage include apnoea and initially increased expiratory phase of respiration. Such cases should be extubated and the tube checked for mucus plugs.
Figure 11.7 Intubation of a Buzzard using a ‘Coles’ endotracheal tube which is tapered at the tip to make intubation easier.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree