Even if surgery is relatively bloodless, there are inevitable losses via the respiratory route because of the drying nature of the gases used to deliver the anaesthetics commonly used in avian surgery. Smaller birds have larger surface areas in relation to volume, and this applies to the lung fields as well as the skin. Avian patients also have an air sac system, which increases the surface area for fluid loss even further.
Many patients are not able to drink immediately after surgery. The period without food or water intake may stretch to a few hours, enough time for any avian to start to dehydrate. Finally, some forms of surgery, such as prosthetic beak repair procedures, will lead to inappetence for a period.
Electrolyte replacement
In cases of chronic fluid loss, electrolytes as well as fluids will often need replacing. Chronic diarrhoea, such as in megabacteriosis or Giardia infestations, will cause loss of food, water and electrolytes to waste. The main electrolyte losses involve bicarbonates and potassium.
Because they have a crop between the mouth and the true stomach, birds may well regurgitate rather than vomit. The crop contents are alkaline to neutral, and so metabolic alkalosis is unlikely to occur with regurgitation or crop problems, indeed metabolic acidosis is more likely. However, in serious proventriculus disease, such as the viral condition ‘macaw wasting syndrome’, stomach megabacteriosis or ulcers, loss of hydrogen ions will occur and metabolic alkalosis can ensue.
Fluids used in avian practice
Lactated Ringer’s/Hartmann’s
This is useful for rehydration and to supply maintenance needs. It is useful for avian patients suffering from metabolic acidosis, e.g. those with chronic gastrointestinal disease and bicarbonate loss, but it can also be used for fluid therapy after routine surgical procedures. The quantity of potassium present in lactated Ringer’s solution is unlikely to cause a problem in birds with hyperkalaemia (such as those suffering from rapid weight loss or with serious skin or tissue trauma). The use of calcium gluconate (5 mg/kg) or the addition of a glucose-containing fluid will help drive the potassium ions into the cells and so reduce the hyperkalaemic threat.
In hypokalaemic birds (such as those suffering from chronic diarrhoea, vomiting, burns or on long-term glucose/saline fluids), the addition of potassium to the fluids at rates of 0.1–0.3 mEq/kg body weight may help stimulate appetite and reduce the risk of cardiac arrhythmias.
In cases of metabolic acidosis, an assessment of bicarbonate ion loss can be made from a blood sample. However, in many cases it is not possible in practice to measure it. Therefore, if persistent vomiting or chronic weight loss or trauma occurs and metabolic acidosis is suspected, a rough approximation may be made. Give a sodium bicarbonate supplement at 1 mEq/kg at 15–30-minute intervals until a maximum of 4 mEq/kg has been reached. (This supplement must not be given with the lactated Ringer’s solution as it will precipitate out.)
Hypertonic saline
This may be used in birds with acute hypovolaemia. It works by rapidly drawing fluid from the cellular and pericellular space into the circulation to support central venous pressure. See chapter 16, Avian Emergency and Critical Care Medicine, for further details of its use. It must be administered intravenously or intraosseously.
Glucose/saline combinations
Glucose/saline solutions are useful for small avian patients. These may well have been through periods of anorexia prior to treatment and therefore may be borderline hypoglycaemic. The concentration to start with when dehydration is present is 5% glucose, 0.9% saline. Once dehydration has been reversed, the avian patient may be moved on to the 4% glucose, 0.18% saline concentration for maintenance purposes.
Protein amino acid/B vitamin supplements
Protein and vitamin supplements can be very useful for nutritional support. Products such as Duphalyte® (Fort Dodge) may be given at the rate of 1 mL/kg/day. They are particularly useful to replace nutrients in cases where the patient is malnourished or has been suffering from a protein-losing enteropathy or nephropathy. They are also good supplements for patients with hepatic disease or severe exudative skin disease such as thermal burns.
Colloidal fluids
Colloidal fluids have been used in avian practice only by the intravenous route, but they can be used via the intraosseous route. They are used in the same way as with cats and dogs:
- When a serious loss of blood occurs
- Where severe hypoproteinaemia is seen
- In order to support central blood pressure
This is due to their ability to stay in the bloodstream for several hours after administration whereas crystalloids remain in circulation for just 15–30 minutes on average. Colloids may be used as a temporary measure whilst a blood donor is selected, or, if a donor is not available, the only means of attempting to support such a patient. Bolus treatment with gelatin colloids such as Gelofusine® (Millpledge) and Haemaccel® (Hoechst) may be given at 10–15 mL/kg intravenously four times over a 24-hour period to aid in the treatment of hypoproteinaemia. Use of Hetastarch® which has a larger colloid particle size and so stays in the circulation for up to 24 hours is preferred where this is available. See section Chapter 16 for Shock fluid therapy.
Blood transfusions
Blood transfusion should be considered if the PCV (packed cell volume) drops below 15%. Birds are more tolerant of blood loss than mammals as the oxygenation of their blood is more efficient. However, transfusions are sometimes required. Donors should be from the same species, e.g. African grey parrot to African grey parrot, or budgerigar to budgerigar. Blood can be collected from the donor into a container or syringe with acid citrate dextrose anticoagulant or, in an emergency, a heparinised syringe may be used. Ideally, a blood filter should be used before blood is transfused into the recipient bird, but frequently this is not possible in general practice, and so collection and administration should be done with care, to reduce haemolysis and clumping.
It is useful to remember that one drop of blood is roughly equal to 0.05 mL and that the estimated blood volume of an avian patient is 10% of its body weight in grams. Volumes that can be transfused range from 0.25 mL in a budgerigar to 5 mL in an African grey parrot.
Oral fluids and electrolytes
Oral fluids may be used in avian practice for those patients experiencing mild dehydration, and for ‘home’ administration. Many products are available for cats and dogs, and may be used for birds. One electrolyte in particular may be useful and that is Avipro® by VetArk. This is a probiotic, but used at the correct concentration may also be used as an oral electrolyte solution. The lyophilised bacteria are useful to aid digestion, which is also often upset during periods of dehydration.
Calculation of fluid requirements
A critically ill avian patient is assumed to be at least 5–10% dehydrated. As with cats and dogs, you should assume that 1% dehydration is equal to the need to supply 10 mL/kg body weight of fluid in addition to maintenance requirements. Assumptions then have to be made on the degree of dehydration of the bird concerned. Roughly,
- 3–5% dehydrated – increased thirst, slight lethargy, tacky mucous membranes, increased heart rate
- 7–10% dehydrated – increased thirst, anorexia, dullness, tenting of the skin and slower return to normal over eyelid or foot, dry mucous membranes, dull corneas, red or wrinkled skin in chicks
- 12–18% dehydrated – dull to comatose, skin remains tented after pinching, desiccating mucous membranes, sunken eyes
These deficits may be large and the volume required for replacement will be difficult to administer rapidly. Indeed, it may be dangerous to overload the patient’s system with these fluid levels all in one go. To spread the deficit evenly, it is advised that the following protocol be used:
- Day 1: Maintenance fluid levels +50% of calculated dehydration factor
- Day 2: Maintenance fluid levels +50% of calculated dehydration factor
- Day 3: Maintenance fluid levels.
If the dehydration levels are so severe that volumes are still too large to be given at any one time, it may be necessary to take 72 hours rather than 48 hours to replace the calculated deficit.
To add to the problem, debilitated avian patients may also be anaemic, and therefore PCVs may appear misleadingly normal, so total protein levels are an additional parameter to look at when assessing dehydration. Uric acid levels may also be measured, as these will often increase in cases of moderate to severe dehydration. Other useful parameters include weight measurement and of course fluid intake and urine output. Table 14.1 gives some normal PCV and total protein values.
Table 14.1 Normal packed cell volumes (PCV) and total blood proteins for selected avian species.
| Species | PCV l/l | Total protein (g/L) |
| Budgerigar | 0.45–0.57 | 20–30 |
| Amazon parrot | 0.41–0.53 | 33–53 |
| African grey parrot | 0.42–0.52 | 26–49 |
| Macaw | 0.43–0.54 | 25–44 |
| Cockatoo | 0.42–0.54 | 28–43 |
| Cockatiel | 0.43–0.57 | 31–44 |
| Mallard duck | 0.42–0.56 | 32–45 |
| Canada goose | 0.35–0.49 | 37–56 |
| Mute swan | 0.32–0.5 | 36–55 |
| Chicken | 0.24–0.43 | 33–55 |
| Pheasant | 0.28–0.42 | 42–72 |
| Pigeon | 0.36–0.48 | 21–35 |
| Peregrine falcon | 0.37–0.53 | 25–40 |
| Barn owl | 0.42–0.51 | 29–48 |
| Tawny owl | 0.36–0.47 | 27–46 |
Equipment for fluid administration
The equipment required to administer fluids to birds is often very small in size. For example, the blood vessels available for intravenous medication are often 30–50% smaller than their cat or dog counterparts and tend to be highly mobile and much more fragile and prone to rupture.
Crop tubes
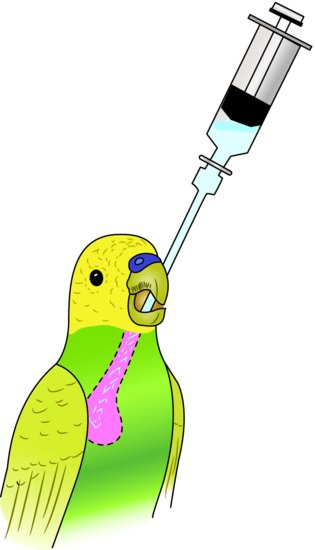
They are useful as a route for fluid and nutrition administration. Crop tubes come either as straight or curved metal tubes, both with blunt ends. To insert a crop tube, extend the bird’s head. Starting from the left side of the inside of the lower beak, pass the tube down the proximal oesophagus into the crop at the right side of the thoracic inlet. Maximal volumes which may be given vary from 0.5 mL in a budgerigar to 15 mL in a large macaw (Figure 14.2).
Figure 14.2 Method of inserting a crop tube. Approach from left side of beak and aim towards the lower right neck region.
Catheters
Because they have a length of tubing attached to the needle, butterfly catheters are extremely useful for the small and fragile avian vessels. If the syringe or drip set is connected to this piece of flexible tubing rather than directly to the catheter, there is less chance of the catheter becoming dislodged; should the bird draw back after the catheter is inserted. Also, the piece of clear tubing on the catheter allows you to see when venous access has been achieved, as blood will flow back into this area without having to draw back on the syringe (which would collapse the fragile veins anyway).
It is advised to flush any catheter with heparinised saline prior to use to prevent clots forming. Twenty-five to twenty-seven gauge sizes are recommended and will cope with venous access for budgerigars through to small conures. Twenty-three to twenty-five gauge sizes will suffice for larger parrots and some of the bigger waterfowl and raptors.
Ordinary over-the-needle catheters may also be used for catheterisation of jugular veins. The latex catheter is useful for long-term maintenance of venous access, as butterfly catheters tend to rupture the vessels if left in for long periods. It is better to use an over-the-needle catheter which has plastic flanges so that it can be sutured to the skin at the site of insertion to prevent removal.
Hypodermic or spinal needles
Spinal needles have a central stylet to prevent clogging of the lumen of the needle with bone fragments after insertion and are therefore useful for intraosseous catheterisation. Twenty-one to twenty-five gauge spinal needles are usually sufficient for most cage birds. Straightforward hypodermic needles may also be used for the same purpose, although the risks of blockage are higher. Hypodermic needles may also be used, of course, for the administration of subcutaneous fluids. Generally, 21–25 gauge hypodermic needles are sufficient for cage birds.
Syringe drivers
For continuous fluid administration, such as is required for intravenous and intraosseous fluid administration during anaesthesia, syringe drivers are becoming more widely used. They are less useful in the conscious bird due to poor tolerance of drip tubing; hence bolus fluid therapy is more commonly used in avian practice.
Collars
It may be necessary to place some of the psittacine family into an Elizabethan-style collar as they are the world’s greatest chewers! There is also a selection of lightweight Perspex neck braces which may be better tolerated. However, these are not so useful when jugular vein catheters are used.
Routes of fluid administration
There are four main routes available for administration of fluids to birds. They are
- Oral
- Subcutaneous
- Intravenous
- Intraosseous
The advantages and disadvantages of the four routes are given in Table 14.2. The intraperitoneal route used in mammals is not available for use in birds, due to the lack of a diaphragm and the presence of air sacs. This means that any injection into the body cavity (or coelom) may inadvertently enter an air sac and thence on to the bird’s airways, resulting in drowning.
Table 14.2 Advantages and disadvantages of various avian fluid therapy routes.
| Route | Advantages | Disadvantages |
| Oral | Reduced stress (if competent handler) Physiological route Less trauma Home therapy possible | Increased stress (if inexperienced) Not useful in cases of digestive tract dysfunction or disease Risk of aspiration pneumonia if regurgitates Slow rate of rehydration (not good for serious hypovolaemia) Inaccurate method of dosing (unless crop tubing) |
| Subcutaneous | Faster uptake of fluids than oral route Volumes given may be large, reducing dosing frequency | Reduced uptake in severe dehydration or peripheral vasoconstriction May be painful in smaller species Only hypotonic or isotonic fluids may be used |
| Intravenous | Rapid rehydration and support of the central venous pressure Use of hypertonic and colloidal fluids possible Good for waterfowl where medial metatarsal veins can take indwelling catheters or multiple venipuncture | Venous access may be difficult in some species Veins may be fragile Some species will not tolerate permanent indwelling intravenous catheters |
| Intraosseous | Rapid rehydration and support of the central venous pressure Useful in smaller species where venous access is difficult May be better tolerated for indwelling catheters than intravenous routes Use of hypertonic and colloidal fluids possible | Potentially painful procedure requiring analgesia and, local or general anaesthetic Risk of bone fracture or osteomyelitis Bolus of fluids takes longer to administer due to rigid confines of bone cortices Avoid use of pneumonised bones (e.g., humerus and femur) as will cause drowning. |
Oral
Lactated Ringer’s solution, probiotic or electrolyte solutions such as VetArk’s Avipro® and Critical Care Formula® or 5% dextrose solutions may be used. The maximum volumes which may be administered via crop tube are given below.
- Budgerigar: 0.5–1 mL
- Cockatiel: 2.5–5 mL
- Conure: 5–7 mL
- Cockatoo: 10 mL
- African grey: 8–10 mL
- Macaw: 10–15 mL
Subcutaneous
Table 14.2 gives the advantages and disadvantages of subcutaneous fluid therapy. The sites for subcutaneous fluid administration are located in the inguinal web of skin which attaches the leg to the body cranially, the axillary region immediately under each wing, and the dorsal interscapular area.
Intravenous
Table 14.2 gives the advantages and disadvantages of intravenous fluid therapy in avian species.
Blood vessels used for intravenous therapy
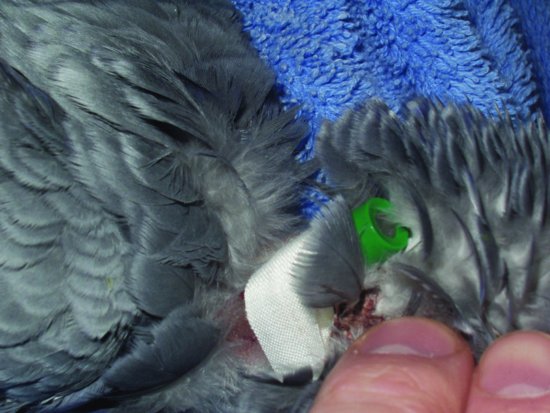
Veins which may be used for intravenous therapy include the basilic and ulnar veins, which run on the underside of the wing in larger species. The right jugular vein may be used for bolus injections in all species down to the size of a canary. In raptors, a jugular vein catheter is very well tolerated for repeated bolus injections (see Figure 14.3). In waterfowl, such as swans and ducks, raptors and some larger parrots, the medial metatarsal vein, which runs along the medial aspect of the lower leg, can be used. Avian species will tolerate catheterisation of this vessel extremely well for several days.
Figure 14.3 A jugular catheter placed in the right jugular of a parrot. Note the silk tape which is then sutured to the bird’s skin to hold the catheter in place. Note also the bung as intravenous fluids in birds are generally given as boluses rather than continuous rate infusion due to the intolerance of drip sets etc.
Volumes of fluid which may be administered intravenously
Isotonic solutions may be given at 10–15 mL/kg per bolus, although volumes up to 30 mL/kg rarely cause problems. Maximum intravenous bolus volumes are given below:
- Finch: 0.5 mL
- Budgerigar: 1 mL
- Cockatiels: 2 mL
- Conure: 6 mL
- Amazon parrot: 8 mL
- Owl: 10 mL
- Cockatoo: 14 mL
- Buzzard: 12–14 mL
- Macaw: 14 mL
- Swan: 25–30 mL
Placement of intravenous catheters
Right jugular vein catheterisation
Medial metatarsal catheterisation for waterfowl
This procedure may be used for larger Psittaciformes and raptors. It may also be performed with the bird conscious, particularly in waterfowl, as the blood vessel is less mobile and likely to rupture.
Intraosseous
Table 14.2 gives the advantages and disadvantages of intraosseous fluid therapy in avians.
Bones used for intraosseous fluid therapy
The two bones most commonly used for intraosseous fluid therapy are the ulna and the tibiotarsus. The ulna may be accessed from a distal or proximal aspect, and the tibiotarsus is accessed from a cranial proximal aspect through the crest just distal to the stifle joint.
Placement of intraosseous catheters
Proximal tibiotarsus
This is the procedure for placing a tibiotarsal intraosseous catheter.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


