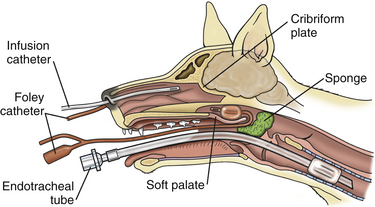
Chapter 65 Aspergillosis is the disease caused by pathogenic fungi that belong to the genus Aspergillus. Aspergillus species are ubiquitous, saprophytic, hyaline molds, the spores of which can be found in soil, water, air, and decaying vegetation. It is estimated that an individual person inhales several hundred Aspergillus spores each day.2 Aspergillus species cause a variety of noninvasive, semi-invasive, and invasive forms of disease in dogs and cats that include keratomycosis, fungal otitis externa, sinonasal aspergillosis (SNA), sino-orbital aspergillosis (SOA), bronchopulmonary aspergillosis, and disseminated aspergillosis. Aspergillus species that can cause disease in dogs and cats are shown in Table 65-1.3–14 Teleomorphs (sexual forms) of the fungus have been identified for some Aspergillus species. Currently, these are named differently and include fungi that belong to the genera Neosartorya, Emericella, and Eurotium. The name Aspergillus may be retained even when teleomorphs are isolated from lesions, to avoid confusion through the use of unfamiliar names.12 Because accurate identification of Aspergillus species (especially rare species) requires advanced molecular techniques, organisms can be misidentified based on their morphology in culture alone. As a result, use of the term “species complex” is recommended when molecular techniques are not used for identification purposes.15 TABLE 65-1 Aspergillus Species Associated with Disease in Dogs and Cats3–14 ∗Some isolates have not been identified beyond the species complex level (including some A. flavus, A. fumigatus, and A. niger). In dogs, SNA is a subacute to chronic, noninvasive disease of the nasal cavity and sinuses. It is most often caused by Aspergillus fumigatus. Rarely, infection by other species, such as Aspergillus niger complex organisms (such as Aspergillus tubingensis) or Aspergillus flavus and the closely related fungus Penicillium, occurs. SNA occurs worldwide. Any breed of dog can be affected, but disease is most common in large, nonbrachycephalic breeds, especially German shepherd dogs, Rottweilers, Border collies, Rhodesian ridgebacks, and retrievers. Dogs of any age can be affected (median age around 6 years).16 No clear sex predisposition exists. SNA is thought to occur when local immune mechanisms fail as a result of uncharacterized genetic or acquired immunodeficiency syndromes, and occasionally as a result of implantation of a foreign body or other preexisting nasal disease, such as nasal neoplasia. Rarely, it occurs in conjunction with other systemic diseases such as hyperadrenocorticism, diabetes mellitus, or systemic autoimmune disorders. However, in most cases, affected dogs are otherwise systemically well and have no evidence of immune compromise. Impaired cell-mediated immune responses have been detected in dogs with SNA, but it is unclear whether this is a cause or consequence of Aspergillus infection.17 Upper respiratory tract aspergillosis is relatively uncommon in cats, and includes SNA and SOA. Approximately 50 cases of URT aspergillosis have been reported in cats to date.3 Fungi that belong to the A. fumigatus complex and, rarely, other Aspergillus species may also be involved (see Table 65-1). A novel organism with a proposed name of Aspergillus felis sp. nov. appears to cause SOA in cats,12 whereas SNA is caused predominantly by A. fumigatus sensu stricto.3 Brachycephalic breeds such as Persians and Himalayan Persians appear to be predisposed to SNA and especially to SOA, but both conditions occur in any breed of cat. Affected cats range in age from 1 to 13 years (median, 5 years). No clear sex predisposition exists.3 Most cats with URT aspergillosis test negative for retrovirus infection; only one cat with SNA in the literature was co-infected with FeLV.18 Five of 8 cats with SNA evaluated at the author’s teaching hospital had diabetes mellitus.4 Aspergillus conidia (or spores), which are 2 to 3 µm in diameter, are inhaled and deposit in the nasal cavity and/or sinuses, where they adhere to epithelial cells. In the absence of adequate immune defenses, the conidia enlarge and germinate to form hyphae. SNA is a noninvasive disease, and the fungus does not extend beyond the mucosal epithelium.19 Production of toxins by the fungus and a profound host inflammatory response is thought to explain the extensive turbinate and bony destruction that follows fungal colonization. Toxins produced by A. fumigatus include aflatoxin, hemolysin, fumagillin, and gliotoxin,2 but the role that these toxins play in canine SNA has not been determined. Gliotoxin induces ciliostasis and apoptosis and can suppress the host immune response.2 The fungus also produces proteases and phospholipases. Chemokines such as Il-8 and monocyte chemoattractant protein-1 (MCP-1/CCL2) are secreted by host inflammatory cells in response to the fungus, which may recruit neutrophils and macrophages to the site of infection.20 Severe disease is associated with destruction of nasal bones, the cribriform plate, and/or the orbit. In cats with SOA, Aspergillus invades the submucosal tissue of the nasal cavity and sinus and extends through the orbital bone and into the retrobulbar space. A profound granulomatous inflammatory response to the fungus results in the formation of a retrobulbar mass, with clinical signs that include exophthalmos, protrusion of the third eyelid, mass lesions in or ulceration of the pterygopalatine fossa, and, rarely, blindness due to invasion of the optic nerve or optic chiasm.3,21–23 Inappetence and dysphagia can also develop.22 Occasionally, the clinical signs of SOA develop after initial signs of SNA, such as sneezing and nasal discharge.3,21 Physical examination findings in dogs with SNA include unilateral or bilateral serous or mucopurulent nasal discharge, epistaxis, pain on palpation of the face, mild mandibular lymphadenomegaly, and sometimes depigmentation and ulceration of the nares (Figure 65-1).24,25 Nasal airflow is usually normal or increased because of turbinate lysis. Serous ocular discharge occurs uncommonly as a result of destruction of the bones of the orbit. Rarely, palpation of the nasal bones or the hard palate can reveal defects in the underlying bone. Dogs with cribriform plate involvement and meningitis may be obtunded or dehydrated. Dogs with severe epistaxis can have evidence of melena on rectal examination secondary to swallowed blood. Most affected dogs are afebrile. FIGURE 65-1 Unilateral depigmentation and ulceration of the nares of a 7-year-old shepherd mix with sinonasal aspergillosis. (Courtesy Dr. Lynelle Johnson, Small Animal Internal Medicine Service, University of California, Davis.) Physical examination findings in cats with SNA consist of serous or mucopurulent nasal discharge, sneezing, stertor, epistaxis, and, in some cases, facial distortion.3 Cats with SOA usually have unilateral exophthalmos and deviation of the globe; bilateral involvement has also been described (Figure 65-2).3,22,23 There may be resistance to ocular retropulsion and protrusion of the third eyelid. Miosis, an absent pupillary light response, and an absent menace response may be present. On examination of the oral cavity, most affected cats also have a mass or ulcer in the pterygopalatine fossa ipsilateral to the affected eye. Other less common findings are exposure keratitis and corneal ulceration, mandibular lymphadenomegaly, pain on opening the mouth, and/or ulceration of the hard palate.3,22 FIGURE 65-2 A, Seven-year-old male neutered Scottish fold cat with exophthalmos, conjunctivitis, and blindness of the right eye secondary to sino-orbital aspergillosis. A 1- to 2-cm mass was also identified in the pterygopalatine fossa on oral examination. B-D, MRI image of the head of the same cat. The right eye was displaced rostrally by a large retrobulbar mass that is mildly, heterogeneously hyperintense on T1-weighted images (B), hyperintense on T2-weighted images (C), and enhanced with contrast (D). The mass extends medial to the ramus of the mandible and enters the nasopharynx as well as the oropharynx. A small T1-hyperintense structure is noted in the left frontal sinus. Dogs with SNA often have no abnormalities on the CBC, serum chemistry profile, or urinalysis. Occasionally, mild nonregenerative anemia; mild neutrophilia, rarely with a left shift; mild eosinophilia; and/or mild hypoalbuminemia are present.4 Cats with SOA may have a mild mature neutrophilia and sometimes mild to severe hyperglobulinemia.3,21 Whenever possible, computed tomography (CT) or MRI are preferred imaging modalities for the nasal cavity because of their increased sensitivity over plain radiography for evaluation of the nasal cavity, sinuses, and cribriform plate.26 Bony changes are best appreciated on CT. Findings on CT or MRI in canine and feline SNA include loss of nasal turbinates and defects in the nasal septum; increased soft tissue opacity within the nasal cavity and/or sinuses due to mucosal inflammation, fluid accumulation, or fungal plaques; hyperostosis of the frontal bones; and mandibular or retropharyngeal lymph node enlargement.3,26–29 Osteolysis of the cribriform plate, orbit, frontal bones, and, rarely, the maxillary or palatine bones may be present (Figure 65-3, C). Meningeal enhancement is sometimes evident on contrast administration in dogs with cribriform plate destruction.25 Fungal plaques and mucosal secretions fail to enhance with contrast and cannot be readily distinguished from each other. In some dogs, lesions are limited to the frontal sinus.27 FIGURE 65-3 Plain radiographs (A and B) and CT scan images (C and D) of the nasal cavity of a 6-year-old female spayed golden retriever with sinonasal aspergillosis. A, Open-mouth view. There is marked loss of detail in the left nasal cavity. B, Skyline projection of the frontal sinuses. There is increased opacity in the left frontal sinus. C, A transverse image through the nasal cavity shows marked turbinate loss on the left side. D, Transverse image through the frontal sinuses and region of the cribriform plate. There is moderate fluid accumulation and irregular soft tissue structures in the left frontal sinus, and the frontal bone that overlies the sinus is thickened and irregular. Bony destruction at the level of the cribriform plate is present (arrow). Advanced imaging of the head in cats with SOA reveals a retrobulbar mass that enhances diffusely or heterogeneously with contrast. Displacement of the globe, osteolysis of the orbit, mandibular lymphadenomegaly, and changes consistent with SNA can also be present. With MRI, retrobulbar masses are T2-hyperintense. Invasion of temporal and masseter muscles by the mass may also be apparent on MRI (see Figure 65-2).30 Plain skull radiographs in dogs with SNA may show unilateral or bilateral loss of turbinate detail, destruction of the nasal septum, and/or increased soft tissue opacity within the nasal cavity. The open-mouth view provides optimum evaluation of the turbinates (see Figure 65-3, A). A “skyline” view of the nasal sinuses may show opacification of one or both sinuses and frontal bone hyperostosis (see Figure 65-3, B).26 Visualization of fungal plaques by rhinoscopy and sinuscopy confirms a diagnosis of SNA. Plaques are white, gray, yellow, black, greenish, or a mixture of these colors and are adhered to the nasal mucosa or mucosa of the frontal sinus (Figure 65-4). Caudal rhinoscopy is performed with a flexible endoscope, after which a rigid telescope or flexible endoscope is used to examine the rostral portions of the nasal cavity. In some cases, extensive turbinate destruction allows a flexible endoscope to be passed directly into the frontal sinuses through the nasofrontal opening. If this is not possible, but CT or MRI findings suggest the possibility of frontal sinus lesions, trephination or surgical exploration of the frontal sinuses is necessary to visualize (and treat) this region. After trephination, a rigid endoscope can be used to examine the frontal sinus through the trephination site. Cytologic examination of blind or rhinoscopy-directed mucosal swabs, brush specimens of the nasal cavity, or nasal biopsies from dogs or cats with SNA can reveal Aspergillus hyphae and sometimes also conidia. Hyphae stain well with Wright’s stains. They are septate and branch at 45-degree angles (see Figure 4-3). Large numbers of degenerate neutrophils, ciliated epithelial cells, and/or bacteria are also usually present. In one study, the sensitivity of cytologic examination was 93% when cytology brush specimens were examined and 100% when squash preparations of biopsies were examined, but it was 20% or less when nasal discharge or smears made from blind swab specimens were examined.31 Aspergillus fumigatus can be readily grown on routine laboratory media. Colonies of mold generally appear within 96 hours, but occasionally longer incubation times are required.32 In the absence of supportive rhinoscopic, cytologic, or histopathologic findings, positive cultures from the nasal cavity (or other nonsterile sites) require cautious interpretation because Aspergillus may be found within the URT of healthy animals. Nevertheless, in two separate studies, no dogs with nonfungal nasal disorders had positive culture results for Aspergillus.16,32 In one of these studies (which included 21 dogs with SNA and 37 dogs with nonfungal nasal disease), the sensitivity of fungal culture of nasal biopsies for diagnosis of canine SNA was 81%.16 In the other study, fungal culture of biopsy specimens or plaque specimens collected by rhinoscopic guidance (≥75%) was more sensitive than culture of blindly collected nasal swab specimens (<20%).32 Growth of an A. fumigatus complex organism from guided aspirates or biopsy specimens collected from a retrobulbar mass in a cat strongly suggests a diagnosis of SOA. In one study, fungal culture of nasal biopsies or aspirates from cats with SOA and SNA had a sensitivity greater than 95%.3 Both serum antibody and antigen assays have been evaluated for diagnosis of URT aspergillosis. Assays for detection of serum antibodies to Aspergillus include ELISA and gel immunodiffusion (ID) assays (see Figure 2-7). Assay types vary in their sensitivity and specificity for diagnosis of SNA, and clinical performance data for some assays used in commercial veterinary diagnostic laboratories may not be available. The sensitivity of one gel ID assay (Meridian Bioscience) for diagnosis of canine SNA was 57% to 77%,16,25,33 and the specificity was at least 98%.16,33 The sensitivity and specificity of another gel ID assay (Immuno-Mycologics, OK) was 31% and 97%, respectively.34 The sensitivity and specificity of an ELISA that detects antibodies against a purified Aspergillus antigen extract was 88% and 97%, respectively.33 Thus, negative results with these antibody assays do not rule out SNA, but positive results strongly suggest the disease is present. The sensitivity and specificity of serum antibody assays for diagnosis of feline URT aspergillosis has not been evaluated in a large number of cats, but both positive and negative assay results have been reported in affected cats.3,28,35 A serum Aspergillus galactomannan antigen ELISA assay (Platelia), originally developed for diagnosis of invasive aspergillosis in humans, has been studied for the diagnosis of SNA and SOA in dogs and cats, but its sensitivity and specificity have been low.5,33,36,37 Only 4 of 17 dogs with SNA were positive in one study, and only weak positive results were obtained (galactomannan indices 0.5-0.8, reference range <0.5).33 False positive results occurred in 11 of 62 dogs without SNA (galactomannan indices of 0.5-1.5). Only 3 of 13 cats with URT aspergillosis tested positive, and false-positive results were obtained in a number of cats without URT aspergillosis (specificity 78%).38 The use of real-time PCR assays that detect Aspergillus DNA in nasal biopsy specimens has been evaluated for diagnosis of SNA in dogs, but assays evaluated had either unacceptably high rates of false-positive test results (PenAsp assay) or a low sensitivity (Aspergillus genus-specific PCR).34 For both assays, dogs with SNA had higher DNA copy numbers than control dogs or dogs with lymphoplasmacytic rhinitis or neoplasia, but there was considerable overlap in copy numbers between dogs with SNA and those without SNA. Histopathology of nasal biopsies from dogs with SNA usually reveals branching, septate fungal hyphae in a background of lymphoplasmacytic and neutrophilic inflammation; occasionally the neutrophilic component predominates. Variable numbers of macrophages and eosinophils may also be present, as well as necrotic debris. Other findings include osteolysis, turbinate remodeling, and intralesional bacteria. Occasionally, intralesional plant material is detected. Mats of fungal hyphae may be present if a plaque is submitted for examination. Conidiophores and conidia can be found because the fungus is exposed to air within the nasal cavity (Figure 65-5). The sensitivity of rhinoscopically guided nasal biopsy for diagnosis of canine SNA in one study was 82% (18 of 22 dogs).25 In that study, three to five biopsy specimens were collected from each side of the nasal cavity. FIGURE 65-5 Histopathology of a nasal biopsy from a 4-year-old intact male Border collie with sinonasal aspergillosis. A variety of noninvasive and invasive protocols for topical antifungal drug treatment of canine SNA have been described with varied rates of success. Currently, there is a lack of masked, randomized clinical trials with consistent and long-term follow-up evaluation that indicate clear benefit of one treatment over another. The protocol described here is used at the author’s institution. A similar protocol has been used successfully to treat SNA in cats.38 A 24F Foley catheter is retroflexed over the soft palate and the balloon inflated until it can be palpated above the soft palate (Figure 65-6). Placement of the catheter is facilitated by insertion of a stiff guidewire into the catheter. The tip of the catheter can then be bent into a U shape and hooked over the soft palate, after which the balloon is inflated. The nasopharynx is packed with moistened laparotomy pads, a gauze pad is placed over the incisive papilla, and the proximal end of the catheter is clamped across the guidewire to prevent leakage of antifungal drug from the nasal cavity. A 10F polypropylene catheter is inserted into each nostril to the level of the medial canthus, or alternatively these two catheters can be placed directly into the frontal sinus with endoscopic guidance.24,39 The tip of an additional 12F Foley catheter is inserted into each of the nares, the balloon inflated, and the catheters clamped so the nares are occluded. Cotton-tipped swabs can also be inserted into the nares to prevent outflow of antifungal drug. Clotrimazole (1%) or enilconazole (1% or 2%) solution is then administered through the polypropylene catheters. For large-breed dogs, 50 to 60 mL of solution is administered per side, and if frontal sinus trephination was performed, half of the solution can be administered into a catheter placed in the trephination site. The patient is rotated 45 degrees every 15 minutes over an hour to distribute the drug into the nasal cavity and sinuses. The catheters and sponges are then removed with the dog’s head tilted downward at an angle of 30 degrees, and the material is allowed to drain from the nasal cavity for 10 minutes. Debridement and a second treatment are then routinely performed 1 month later; sometimes, a third treatment may be necessary. Clotrimazole can be irritating to tissues, and solutions that contain propylene glycol can cause pharyngeal irritation and edema.40 Enilconazole solutions may be less irritating, but there is no evidence that they are more effective than clotrimazole. FIGURE 65-6 Diagram illustrating placement of catheters for topical treatment of canine sinonasal aspergillosis with clotrimazole or enilconazole.
Aspergillosis

Upper Respiratory Tract Aspergillosis
Clinical Features
Physical Examination Findings

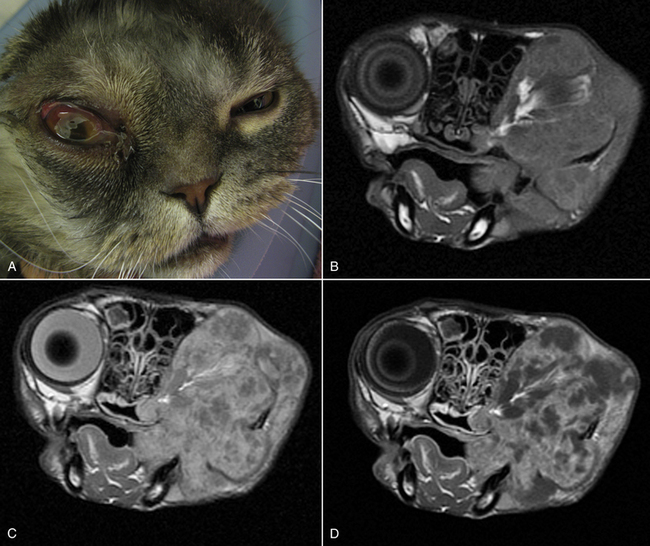
Diagnosis
Laboratory Abnormalities
Diagnostic Imaging
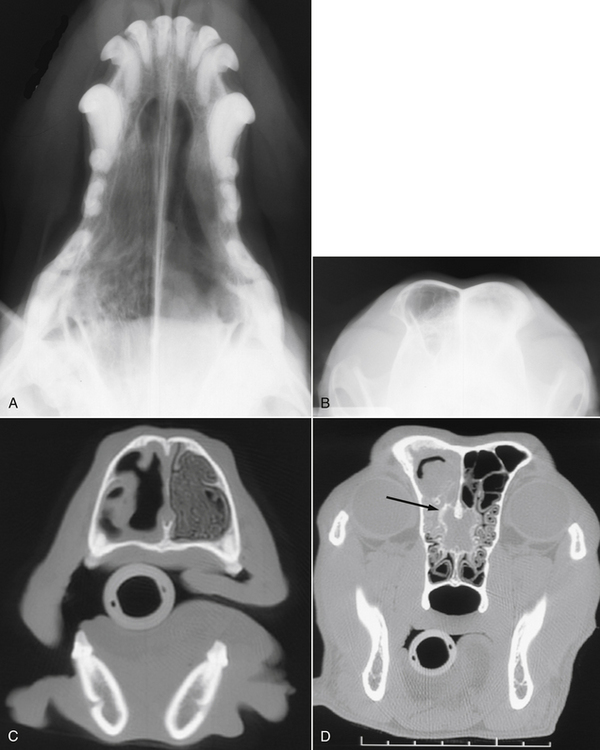
Plain Radiography
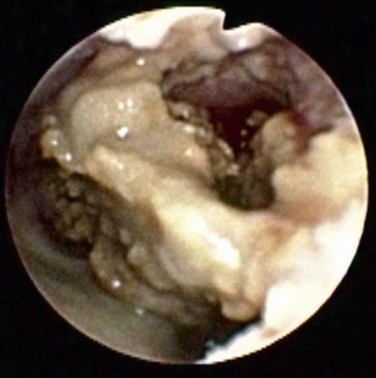
Rhinoscopy and Sinuscopy
Microbiologic Tests
Fungal Culture
Serologic Diagnosis
Molecular Detection Using the Polymerase Chain Reaction
Pathologic Findings
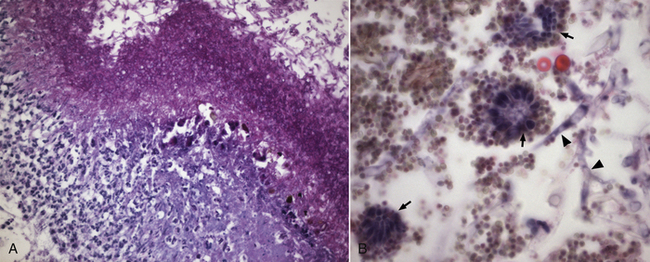
A, There is a profound neutrophilic inflammatory response beneath a thick mat of fungal hyphae, which stain magenta. Periodic acid–Schiff stain, 400× magnification. B, Aspergillus hyphae (arrowheads), conidiophores (small arrows) and conidia (spores) can be seen on the surface of the lesion. H&E stain, 1000× oil magnification.
Treatment and Prognosis
Topical Antifungal Drug Treatment
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Aspergillosis
Only gold members can continue reading. Log In or Register to continue