Chapter 15 ARTIFICIAL INSEMINATION WITH FROZEN SEMEN
Pregnancy rates of mares bred with frozen semen are, on average, around 50%.1,2 However, it is becoming more obvious that the quality of the frozen semen that is commercialized is improving and pregnancy rates are steadily increasing with the majority of stallions that are offered in the open market. This is perhaps due to the improvement in the freezing techniques and the freezing extenders used. In addition, it is almost standard that frozen stallion semen is now exclusively packed in 0.5-ml straws. Although it is evident that the average semen quality and the fertility are improving, one has to look at averages with a certain degree of caution since these numbers can sometimes be misleading. It is well accepted that although an average pregnancy rate per cycle with frozen semen is around 40% when properly used,3 there is significant variation in the results, and it is not uncommon to have per cycle pregnancy rates ranging between 0% to over 70%.4 In addition to this variation, there is a lack of standardization in the information that is available regarding time of an insemination and semen handling procedures.5,6 Individuals involved in the insemination should know the factors that affect the fertility of frozen semen, so that they can convey realistic expectations to the stallion and mare owners. These factors are (1) the stallion and its semen quality, (2) the fertility of the mare, and (3) the reproductive management and the handling of the semen.
STALLION FACTORS
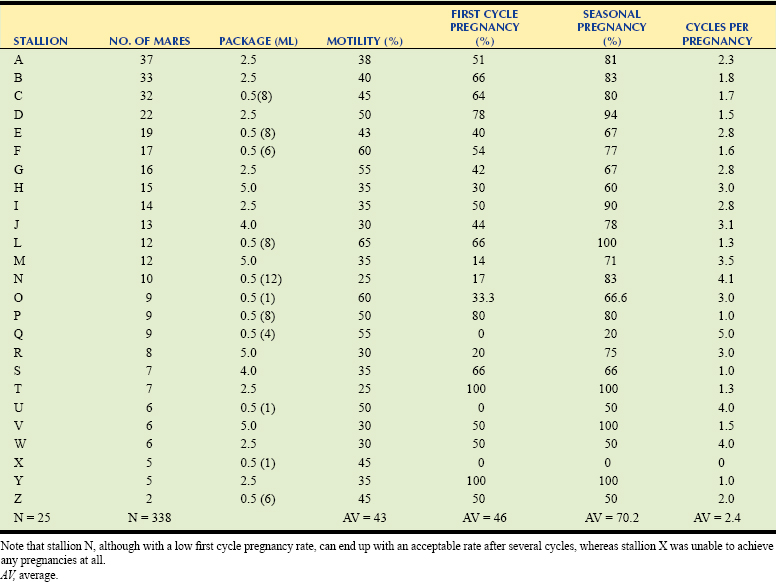
Thousands of foals are born every year using frozen, thawed semen. Although the technology is still far from optimal, it has gained, and is still gaining, wider acceptance in the industry. An increasing number of stallion and mare owners are taking advantage of the benefits of using frozen semen. Perhaps one of the most important reasons why frozen equine semen is still far from optimal is because there is wide variability in the ability of semen from individual animals to tolerate the freezing and thawing process. It is thought that only 25% of stallions will achieve pregnancy rates compared with those of stallions used for fresh semen or natural cover when inseminated into healthy mares at the proper time.6 Although the other 75% of the stallions will produce suboptimal pregnancy rates on a per cycle basis, end-of-season pregnancy rates for some of these stallions can reach more than 80%.7 The drawback is that the number of breeding cycles needed to achieve acceptable pregnancy rates is often too high, making this technique too expensive for the mare owner. Table 15-1 shows the variability in pregnancy rates obtained with frozen semen from different stallions when bred at the uterine body.
Table 15-1 Variation in First Cycle and Seasonal Pregnancy Rates of Stallions Used with Frozen Semen and Deposited in the Uterine Body∗
It is still uncertain why there is so much variation in the ability of semen to tolerate the freezing and thawing process. Among other reasons, constituents of the seminal plasma or molecules of the sperm itself may be responsible. But stallion owners must also realize that the selection criteria for stallions rarely, if ever, involve semen quality or fertility. Therefore, breeding to stallions that have poor semen could affect the semen quality of the offspring. It is well accepted that there are sire lines known for poor semen quality as well as others known for good quality.8 There are also sire lines known for good frozen semen quality.
Previous work has shown that this inherent stallion variability is one of the most important factors in determining the pregnancy rates with frozen, thawed semen.3 Because sperm from individual males responds differently to the freezing and thawing process, several investigators and laboratories have tried numerous systems to try to improve the survival of equine sperm after thawing.9
It is still well accepted that one of the most important factors affecting the quality of frozen stallion semen is the quality of the raw ejaculate.10 The technique for semen collection, processing, and extender type is perhaps just one of the factors. However, if the quality of the raw ejaculate is an important factor in determining the post-thaw quality, it seems that sperm should not be collected for freezing while the stallion is sexually rested. Collection of at least two to four ejaculates before the first freezing should be done to maximize the quality of the raw semen. This procedure should be done even when the semen apparently looks to be of good quality under the microscope.
Recent experimental data have indicated that stallions with apparent suboptimal semen quality for cooling and freezing can be moderately improved by increasing the levels of omega fatty acids in their diet.11 In addition it is very important that the stallion be in a stress-free environment with the ability to be exercised.
SEMEN PROCESSING
The protocol for processing semen for cryopreservation (freezing) involves the following:
Once the stallion’s extragonadal reserves are stabilized and a detailed evaluation has been previously performed, the evaluation of the raw semen immediately prior to cryopreservation includes the estimation of motility and the calculation of the total number of sperm in the ejaculate. Previously centrifugation extender consisted of mostly a glucose-EDTA solution.12 Currently it is almost standard practice that the raw semen is quickly diluted at a ratio of 1:1 with any of the milk-based extenders described in Chapter 14.
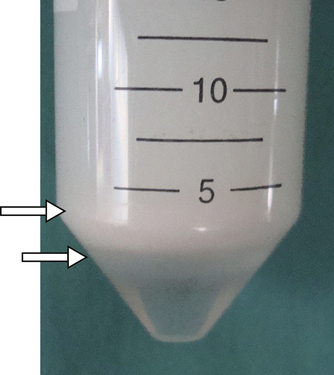
The centrifugation process has been significantly improved in recent years. The addition of a high-viscosity carbohydrate cushion to the bottom of the centrifugation tube prevents the sperm pellet from compacting against the bottom of the tube (Fig. 15-1). The quality of the sperm pellet is also a critical aspect of the process of semen processing for cryopreservation. The advantage of using the sperm cushion includes a higher recovery of sperm due to higher gravitational centrifugation force (800–1000× g) compared with the centrifugation force of 300–500× g) for semen without a cushion.13 Commercial cushion preparations are available from MiniTube or IMV.
Although it has been argued that differences in the composition of the extender will improve the quality of frozen-thawed semen from certain stallions, experimental data have not supported this concept. Stallions considered to be “good freezers” based on post-thaw motility and/or pregnancy rates were not affected by extender type, whereas semen from stallions whose semen did not freeze well could not be improved by simply changing the extender.14 However, it must be noted that most of the experiments done with changing the extender composition have used 3%–5% glycerol as the cryoprotectant. Recent evidence has shown that a combination of glycerol with methylformamide has significantly improved post-thaw motion characteristics as well as pregnancy rates of frozen thawed semen.15 Preliminary results16 have also indicated that the use of DMSO as a cryoprotectant and L-ergothioneine as supplement and antioxidant or glutamine to the extender has the potential of improving motion characteristics of sperm after thawing.17 The amount of freezing extender added to the pooled sperm pellet should be no less than two thirds of the total volume and the practitioner must be careful of not overextending the semen before packaging.
Semen for freezing has been placed in a variety of packages including 5.0-, 4.0-, 2.5-, 0.5-, and 0.25-ml straws, aluminum tubes, glass ampoules, glass cryovials, and polyethylene bags.18 The current standard package is the 0-5-ml straw with 500-800 million total motile sperm loaded in one or up to eight straws per insemination dose prior to freezing.
The freezing process starts by an initial cooling of the semen at 0.5°C/min until 6°C. Once that temperature has been reached, a 30- to 60-minute equilibration time is done. Freezing rates for 0.5-ml straws vary between stallions and range from −15°C to −40°C/min until −130°C, at which time the straws are plunged into the liquid nitrogen. Semen can also be frozen successfully by placing the loaded straws on a rack horizontally 2.5–4 cm above the liquid nitrogen. A recent report has also shown that straws can be frozen in plastic goblets containing 5 × 0.5 straws, avoiding further manipulation of individual straws.19
It has been shown experimentally that the raw semen from stallions that are considered “good freezers” have lower volumes, higher sperm concentrations, and higher sperm motility compared with semen from those classified as “poor freezers.”20 In contrast to these results, Brinsko et al.21 reported a lack of correlation between raw semen parameters and those of cooled stored or frozen semen. Selection criteria of these stallions could explain some of the conflicting results found by these two groups.
The standard procedure has been to collect the stallion and freeze semen at a location where the equipment and technology are centralized or to have a mobile unit that will go to the stallion location. One report indicates that it is possible to ship the stallion’s entire ejaculate overnight to a central location in order to be frozen with the appropriate technique. Although not ideal, it could solve the problems of stallions that are located in remote areas.22
Freezing of semen from stallions that have suddenly died can be performed by shipping the testicles and epididymides in a cool pack to a central location. Freezing of epididymal semen with extenders containing a combination of glycerol and formamide as cryoprotectant seems to optimize the results.23,24
Post-Thaw Evaluation
Post-thaw evaluation of frozen semen is a difficult and controversial topic. Evaluation is generally based on sperm motility, longevity of motility, and morphology. It is thought that frozen, thawed semen having longer longevity tends to produce better pregnancy rates. It is becoming a routine procedure to evaluate the longevity of thawed semen at 37°C, 20°C, or 5°C. Motility, besides being a fairly subjective measure of quality, is a poor predictor of fertility.14 Hence, it is important for veterinarians using frozen semen routinely to evaluate the morphology of the sperm. It is not uncommon to have stallion sperm with fairly good motility and poor morphology, which could account for some sperm with poor fertility despite good motility after thawing. Bioassays such as zona binding or oviductal epithelial binding may prove to be better indicators of sperm viability and potential for fertilization.25,26
Although there are no standards, minimum criteria required for semen to be used commercially seem to be 30%–35% progressively motile with >600 million total sperm per insemination dose. With a small group of mares, Squires et al.27 recommend that a dose of 250 million progressively motile sperm optimizes fertility when semen was inseminated in the uterine body. Furthermore, Colorado investigators recently reported that stallion semen frozen in 0.5-ml straws that had 320 million progressively motile sperm after thawing resulted in higher pregnancy rates.28 There is still no consensus on the minimum number of progressively motile sperm per dose needed to maximize fertility; this perhaps is a stallion-dependent factor, as has been reported for the bull. Semen frozen in The Netherlands needs to meet a minimum number of 300 million progressively motile and morphologically normal sperm in order to be sold commercially. However, the site of deposition of the semen could influence the pregnancy rates dramatically. A detailed discussion can be found later in this chapter.
For each of the cryopreservation steps previously mentioned, there are a number of alternatives and variations that are used.29 This has resulted in a great number of variations in the techniques and in the results of the quality of frozen-thawed semen.
For a good percentage of the stallions, the process of cryopreserving semen results in some degree of reduction in their fertility. This is due to the effect of freezing and thawing on some of the characteristics that sperm must retain to be able to achieve fertilization. Spermatozoa that have been frozen have an impaired ability to reach the oviduct or site of fertilization. Fewer sperm reach the site of fertilization, or perhaps sperm take a longer time to reach it.30 Sperm attach to the oviductal epithelium through a complex interaction of the carbohydrates present on the plasma membrane.31
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree