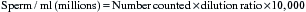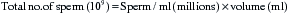Chapter 14 ARTIFICIAL INSEMINATION WITH FRESH AND COOLED SEMEN
Even though the mare’s fertility and her reproductive management are key factors in the establishment of pregnancies, supplying semen of optimal quality for artificial insemination (AI) is imperative. All semen collection procedures described in Chapter 3 can result in samples that are adequate for AI. However, semen must be collected, handled, and processed adequately in order to maintain the fertility of the spermatozoa. Breeders, farm managers, and veterinarians often perform a cursory analysis of semen that will be used for fresh or cooled AI, but depending on the intended use, semen must be handled differently. This chapter will describe the handling procedures of stallion semen that is going to be used within a few hours and up to 48 hours after collection.
There are many ways and reasons for semen evaluation. A lengthy discussion of a complete semen evaluation can be found in Chapter 6, but a very simple semen evaluation should always be done when semen is going to be processed. However, the most important factor is the proper handling of the raw semen in order to avoid artifacts or iatrogenic damage to the sperm that could affect the results and the calculations performed for the estimation of a breeding dose. Sperm concentration and sperm motility are perhaps the two key factors that must be evaluated accurately in order to properly prepare breeding doses.
HANDLING OF RAW SEMEN
Immediately after collection, raw semen must be maintained at body temperature (35°–38°C), and so does the equipment that will come in contact with it. Therefore, it is necessary and highly recommended for laboratories that process semen to have a 37°C incubator available. Sudden temperature changes will cause temperature shock, causing irreversible and detrimental changes to sperm. Thermal shock is a sudden temperature reduction of more than 8°–10°C to sperm cells and can cause cold shock. This irreversible damage to sperm is evidenced microscopically by a typical forward or backward tight circular motility pattern of the spermatozoa. Cold shock also affects the longevity as well as the fertilizing potential of the sperm. The reduction in fertilizing potential, as well as the abnormal motility observed, is a consequence of the reduction in the fluidity of the lipid component of the plasma membrane.1 The rigidity of the structure of the membrane caused by this sudden change makes the sperm less able to accommodate to volume changes when exposed to different osmotic pressures. The reasons for cold shock include cold incubators, cold receptacles for semen collection, cold slides and cover slips, or cold ambient temperatures without proper protection of the semen.
Although it does not happen as often as cold shock, exposure of sperm to temperatures over 10°C above body temperature for short periods will also cause irreversible changes to sperm. Heat shock is often evidence under the microscope by a change in the motility pattern from a linear and progressive motion to a small circular tight pattern. Longevity of sperm motility is also significantly reduced. Heat shock is most often induced by either exposure of semen to high temperatures in the artificial vagina liner, by accidentally having incubators or slide warmers too hot, or by prolonging the thawing time of frozen semen longer than what is recommend when temperatures are above 37°C. Another type of shock is the osmotic shock, which can be induced by exposing the sperm to hyperosmotic (>400 mOsm) or hypo-osmotic (< 250 mOsm) environments. This type of shock can be induced when extenders are improperly made or have too little or too much water, respectively. Hypo-osmotic shock can be shown by the typical swelling of the plasma membrane, resulting in a characteristic coiling of the sperm tail. Hyperosmotic shock can be induced by the leaking of inappropriate artificial vagina lubricants into the semen receptacle, resulting in a significant reduction in the longevity of motility of the spermatozoa.2 Hypo-osmotic shock can be evidenced by a typical coiling of the tail either as hair pins or a complete wrap around the sperm head as the plasma membrane swells, drawing water to the inside of the cell.
SPERM CONCENTRATION
Automated Counters
A recent study by Texas workers using the hemocytometer as the gold standard concluded that all types of concentration calculating devices that are based on semen turbidity are very accurate over a small range of sperm concentrations (150–300 million/ml). However, sperm concentrations lower than that were overestimated while higher concentrations were underestimated.3 Recently, a new sperm counter known as the NucleoCounter SP-100 (Allerod, Denmark) uses fluorochromes to stain sperm and therefore appears to be able to differentiate sperm from other particles. Since it detects sperm nuclei and is not based on turbidity, the NucleoCounter SP-100 can be used to count sperm in the presence of extenders.4
SPERM MOTILITY
Computer-Assisted Semen Analysis
In order to increase the objectivity of motility readings, there are several computerized motility analyzers.5 This equipment, although very costly, will provide information such as velocity of sperm, amplitude of lateral head displacement, and other parameters that would be very difficult to assess with a regular microscopic evaluation. Since the computer analyzes particles the size of a sperm head, when semen is extended and evaluated with the motility analyzer, one has to ascertain that particular matter in the extender does not interfere with the motility estimates. Although computer-assisted semen analysis (CASA) systems are commonplace in most human and andrology laboratories, its use in veterinary medicine has been restricted to a few laboratories because of the cost of the equipment. However, semen samples can be videotaped and sent to referral laboratories where the analysis can be performed. A more detailed discussion is found in Chapter 6.
Morphological Evaluation
Numerous studies have tried to explain different levels of fertility by correlating the number of normal or abnormal sperm present in an ejaculate. Jasko et al.6 reported that the number of abnormal heads, midpieces, and proximal droplets accounts for 60% of the variation in stallion fertility.
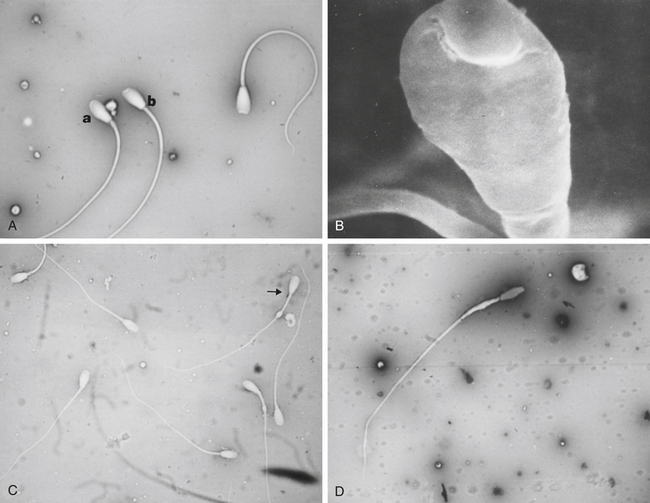
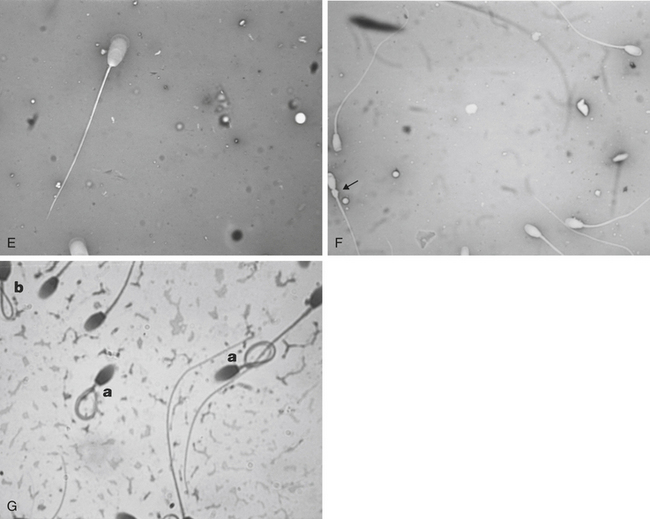
Morphological evaluation of sperm cells can be performed by evaluating at least 100 cells on a stained smear with the oil immersion objective (100×). Routine stains used for this purpose include: eosin nigrosin, aniline blue, or India ink.7 However, if the sperm have been diluted with an extender, it is necessary to dilute the sample in 10% buffered formal saline or glutaraldehyde to fix the sperm. Evaluation will be done on a wet sample with the oil immersion objective and a phase contrast microscope. The morphological categories that should be recorded include the number of normal sperm, abnormal heads or nuclei, abnormal acrosomes, abnormal necks, tailless heads, proximal droplets, distal droplets, abnormal midpieces, abnormal tails, and round spermatids (cells).
Fig. 14-1 shows some of the most common morphological features observed in stallion spermatozoa. Sperm cells that have more than one defect should be recorded and the frequency of them noted on at least 100 cells. It is important to realize that the morphological evaluation should record the frequency of defects in at least 100 cells and not the number of abnormal cells out of 100. It is not clear how an individual sperm abnormality affects the fertility of a stallion. It can be assumed that stallions with a higher number of sperm abnormalities will have lower fertility compared with one with higher numbers of morphologically normal sperm. However, the different levels of fertility reduction are not well established. Sperm abnormalities are the result of testicular or epididymal insult.8,9 A slight increase in bull’s testicular temperature (3.1°C) for 48 hours causes a significant increase in sperm defects that are evidenced as early as 8 days after. This is followed by a peak in abnormalities around 18 days. Furthermore, after 39 days the animals in this study still had not regained their pre-treatment morphology.9 Interestingly, in this study sperm motility was only slightly and transiently affected. The transient effect of increased testicular temperature had no effect on sperm output. The authors suggest that the type of defect depends on the stage of the spermatogenic cycle where the cell was at the time. In other words, when sperm with increased cytoplasmic droplets were at the epididymal level, tailless heads were in the process of spermiation, nuclear vacuoles were germ cells in early stages showing a lack of DNA condensation, and head changes would be inflicted to spermatids that were elongating. Stallions are exercised and competed during the hot summer months, which could have a significant impact on sperm morphology, particularly on stallions with both duties (performance and breeding).
High testicular temperatures can also be self induced by stallions that carry their testicles high in the inguinal area during periods of stress, pain, or competition. The morphology of the sperm cells can be affected not only by high scrotal temperatures, but also by prolonged stress during training of competition, exogenous hormones such as progesterone or anabolic steroids, and sexual rest.9–11 The individual stallion’s susceptibility to factors that affect sperm morphology remains to be investigated.