Fig. 21.1
Confocal laser scanning micrographs showing the distribution of endogenous albumin (red color) and IgG (green color) in living mouse glomeruli under four different fixation methods. (a) Both albumin and IgG were exclusively colocalized along or within the glomerular capillary loop s (GCLs) with the “in vivo cryotechnique ” (IVCT), (b) under quick-freezing following resection (QF), and (c) conventional immersion-fixation (IF); (d) we found both albumin and IgG in Bowman’s space. Under quick-freezing following perfusion -fixation (PF), we detected a weak indication of both albumin and IgG in the GCLs, but more clearly detected these proteins in Bowman’s space and on apical cell membranes of proximal convoluted tubule s . Scale bars 10 mm
Under the normotensive condition by “in vivo cryotechnique ,” the immunofluorescence for albumin and IgG was exclusively detected in GCL with little colocalization with the PAS fluorescence emission, and IgG was also at MA. To the contrary, under the quick-freezing of resected kidney tissues or with immersion-fixation condition, both of the albumin and IgG immunoreactivity were also detected in Bowman’s space. These findings suggest that some amounts of albumin or IgG, which are almost kept within GCL under the normotensive condition, easily pass through the GBM and translocate into Bowman’s space under the quick-freezing of resected kidney tissues or immersion-fixation condition (Fig. 21.2).
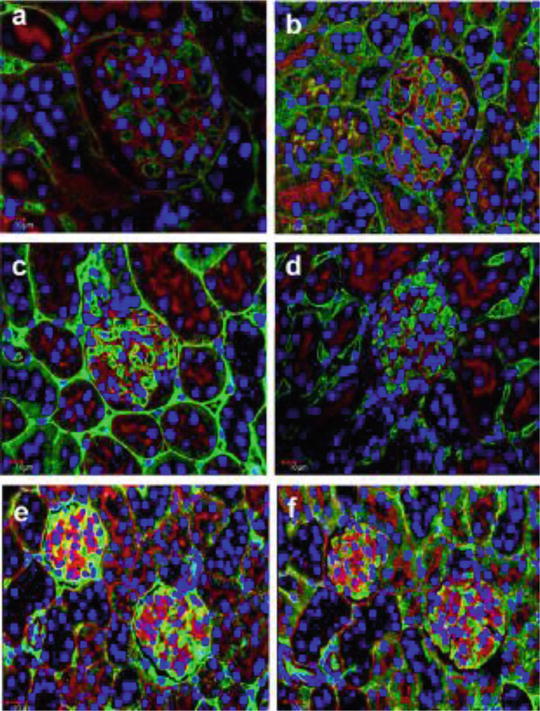

Fig. 21.2
Confocal laser scanning micrographs showing albumin or IgG (green color) passing through GBM filtration barriers under various fixation conditions using the PAS fluorescence emission (red color) as a marker for the GBM. With the IVCT (a, b), the immunofluorescence for albumin and IgG was exclusively detected in GCLs with little colocalization with the PAS fluorescence emission, and IgG was also in mesangial areas (b). After QF (c, d) or IF (e, f), both albumin and IgG immunoreactivities were detected in Bowman’s space on the opposite side of the GCLs and bordered by the PAS-positive GBM. The overlapping images of albumin or IgG immunoreactivity with PAS fluorescence emission, represented by a yellow color, was more widely apparent with IF (e, f) than with QF (c, d). Scale bars 10 mm
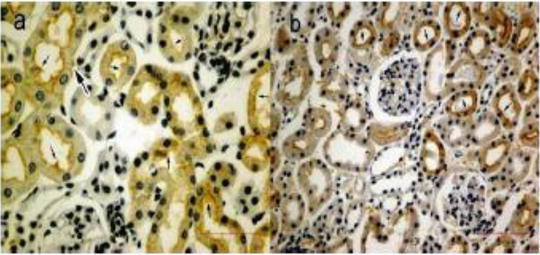
21.3 Metal-Enhanced 3,3′-Diaminobenzidine (DAB) Staining of Collagen Type IV and Aquaporin-1(AQP-1 ) in Living Mouse Glomeruli and Proximal Tubules
The common HE staining was performed to examine native morphology in the living mouse kidney s , as obtained by the “in vivo cryotechnique .” Well-preserved areas of renal cortices under normotensive conditions could be obtained within 300–400 μm from the frozen surface tissues without visible ice crystal s at a light microscopic level, and the GCLs are kept open. Although collagen type IV is reported to be clearly immunolocalized in the GBM and MAs after a conventional fixation method, when we employed the “in vivo cryotechnique ,” we found marked immunolocalization of collagen type IV in the MAs, but only weakly immunolocalized on the GBM. To visualize the expression of water channel AQP-1 in living mouse proximal tubule s , the immunostaining for AQP-1 was performed, respectively, on paraffin section with “in vivo cryotechnique” and perfusion-fixation methods. Using perfusion-fixation method, the immunoreaction product of AQP-1 was observed clearly along the basolateral membrane of proximal tubule, and its distribution was disorganized and irregular on the brush border and apical cell membrane along the proximal tubule due to the artificial state (Fig. 21.3).