Chapter 35 Antiemetic Agents
Vomiting or emesis is a complex reflex pathway that has evolved to protect animals from ingested toxins, but it holds greater importance because of the large number of medical conditions that may cause or be associated with it.1,2 Emesis may occur with such diverse conditions as systemic (e.g., septicemia, multiple organ failure), metabolic (e.g., uremia, liver failure), and endocrine (e.g., hyperthyroidism, hypoadrenocorticism) disease, as well as with the inflammatory, infectious, neoplastic, obstructive, and toxicologic disorders of the primary gastrointestinal tract, pancreas, liver, and biliary tract. In each of these circumstances, vomiting is believed to be activated through a neural or humoral mechanism.3,4
Physiology of Vomiting: Humoral and Neural Pathways
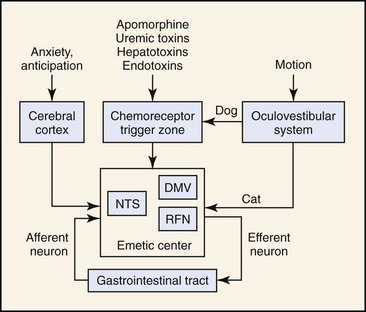
The first mechanistic studies of vomiting were carried out by Borison and Wang in the early 1950s.3,4 Those authors postulated a two-component model of vomiting involving activation of a humoral or neural pathway. In the Borison-Wang model, vomiting was postulated to occur through activation of the chemoreceptor trigger zone (CRTZ) by bloodborne substances (humoral pathway), or through activation of the emetic center by vagosympathetic, CRTZ, nucleus tractus solitarius, vestibular, or cerebrocortical neurons (neural pathway). Through a complicated series of experiments, Borison and Wang showed that activation of the CRTZ by circulating emetogenic substances (e.g., uremic toxins, cardiac glycosides, endotoxins, and apomorphine) could be abolished by CRTZ antagonism, but not by vagotomy, sympathectomy, or emetic center antagonism. At the same time, neural activation of the emetic center by gastrointestinal disease (e.g., inflammation, infection, obstruction, toxicity) could be abolished by vagotomy, sympathectomy, and emetic center antagonism, but not by CRTZ antagonism. Thus, vomiting was most easily explained by two independent mechanisms: one mechanism involving an essentially neural pathway, the other mechanism operating through a humorally dependent pathway.3,4 The Borison-Wang two-component model has helped to explain many of the vomiting disorders of animals and humans. The model is not without challenge, however, and it has been suggested that there are parallel mechanisms for the initiation of emesis in response to any stimulus, and it is the sum of the inputs that drives the emetic response.5 In other words, emesis need not be simply an either/or response. The concept of a discrete emetic center has also been seriously challenged. Based on more recent electrophysiologic studies, a model of sequential activation of a series of effector nuclei (e.g., nucleus tractus solitarius, retrofacial nucleus, dorsal motor nucleus of the vagus) has been proposed that doesn’t require a discrete emetic center.5 Despite contemporary reexamination, there still is good agreement on two general patterns of emesis, one humoral and the other neural (Figure 35-1).
The essential component of the humoral pathway is the CRTZ located within the area postrema, which is sensitive to activation by bloodborne substances. The CRTZ is located anatomically outside of the blood–brain barrier and is readily perfused by substances in the systemic circulation. Receptors within the CRTZ may be activated by many endogenous (e.g., uremic-, hepatoencephalopathic-, or endotoxins) or exogenously derived (e.g., digitalis glycosides, cis-platinum, apomorphine) bloodborne substances. Many pharmacologic approaches to antiemetic therapy are based on receptor interactions at the CRTZ, emphasizing the humoral pathway of emesis.1,4
Although many antiemetic agents are based on CRTZ pharmacology, many spontaneous vomiting disorders result from activation of the neural pathway. Vomiting associated with primary gastrointestinal tract disease (e.g., inflammation, infection, malignancy, toxicity) results from activation of an afferent neural pathway, nucleus tractus solitarius neurons, and the emetic center. Efferent information transmitted back to the gastrointestinal tract stimulates the motor correlates of vomiting; that is, retrograde duodenal and gastric contractions, relaxation of the gastroesophageal sphincter, gastroesophageal reflux, opening of the proximal esophageal sphincter, and evacuation of gastrointestinal contents.7 A neural pathway can also be involved in vomiting associated with motion sickness. Although there are important species differences,8–10 motion within the semicircular canals is transduced to vestibulocochlear neurons that ultimately synapse in the CRTZ (dog) or emetic center (cat). Finally, a neural pathway involving cerebrocortical neurons is very likely involved in vomiting disorders associated with anxiety or anticipation, but its importance in companion animals has not yet been established.
Pharmacology of Vomiting: Neurotransmitters and Receptors
Chemoreceptor Trigger Zone
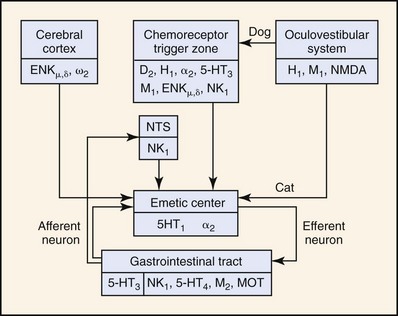
Neurochemical studies have demonstrated the presence of several neurotransmitters: dopamine, norepinephrine, 5-hydroxytryptamine (5-HT, serotonin), acetylcholine, histamine, substance P, and enkephalins; their respective receptors or binding sites: D2 dopaminergic, α2 adrenergic, 5-HT3 serotonergic, M1 cholinergic, H1 and H2 histaminergic, NK1 neurokininergic, and ENKµ and ENKδ enkephalinergic; and their respective synthetic or degradative enzymes: DOPA (dihydroxyphenylalanine) decarboxylase, dopamine β-hydroxylase, 5-hydroxytryptophan decarboxylase, choline acetyltransferase, histidine decarboxylase, aminopeptidase N, and enkephalinase (Figure 35-2).11 Some neurotransmitter-receptor signal transduction pathways are probably more important than others. For example, apomorphine, a D2-dopamine receptor agonist, is a potent emetic agent in the dog, but it does not readily induce emesis in the cat.12 This finding has two important implications: (a) CRTZ D2-dopamine receptors may not be as important in mediating humoral emesis in the cat, and (b) D2-dopamine receptor antagonists (e.g., metoclopramide) may not be as effective as an antiemetic agent in the cat. Xylazine, an α2-adrenergic agonist, is a more potent emetic agent in the cat than in the dog.12,13 Xylazine’s effect suggests that α2-adrenergic antagonists may be more useful antiemetic agents than D2-dopamine antagonists in the cat.9 Cancer chemotherapy (e.g., cis-platinum, doxorubicin, cyclophosphamide)-induced emesis is mediated by activation of 5-HT3 receptors in the CRTZ of the cat,11,14–16 while visceral and vagal afferent 5-HT3 receptors may be more importantly involved in the dog.17 Antagonists of the 5-HT3 receptor are efficacious in the prevention of emesis associated with cis-platinum and other chemotherapy in cats14–16 and dogs.18 NK1 receptor antagonists represent a unique new class of antiemetic agents that are based on substance P pharmacology at the CRTZ, as well as the nucleus tractus solitarius. This group of antiemetic agents may have advantages over other antiemetic classifications in that they may inhibit vomiting through both pathways (humoral and neural).19,20 Finally, although histamine and H1– and H2-histaminergic receptors have been demonstrated in the CRTZ of the dog, they have not yet been demonstrated in the cat. Histamine is a potent emetic agent in the dog, but the cat seems resistant to its emetic effects.11,12 H1-histaminergic antagonists (e.g., diphenhydramine, dimenhydrinate) are ineffective antiemetic agents for motion sickness in the cat (Table 35-1).10
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree