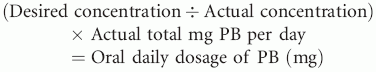Chapter 186 Anticonvulsants
• The first-line drug for control of status epilepticus is diazepam. In animals refractory to this drug, a midazolam or propofol bolus and infusion are administered.
• Oral phenobarbital (PB), the first-line antiepileptic drug (AED) for chronic seizure control in dogs and cats, is typically titrated to clinical effect and therapeutic serum levels (20 to 40 μg/ml in dogs; 10 to 20 μg/ml in cats).
• Potassium bromide is an effective second-line AED in dogs and cats, although its use is limited in cats because of the risk of inducing severe lower airway disease in this species.
• In contrast to its effect in dogs, oral diazepam is effective for long-term control of seizures in cats. However, the risk for liver failure after oral, but not intravenous, administration restricts its use in cats.
• Newer AEDs exert pharmacokinetic properties associated with little or no drug-drug interaction. Limited clinical data suggest potential benefit as add-ons or monotherapy in small animals.
INTRODUCTION
• Status epilepticus: a continuous, generalized, convulsive seizure lasting more than 5 minutes, or two or more seizures between which no normalization of mental status occurs
• Refractory status epilepticus: seizures lasting more than 2 hours or more than two seizures per hour without normalization of mental status, despite standard antiepileptic treatment1 (see Chapter 98, Seizures and Status Epilepticus)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree