Chapter 187 Anticoagulants
• Thromboembolic complications may be a significant contributor to morbidity and mortality in critically ill patients.
• Anticoagulant therapy has its effect by altering only one aspect (blood factors) of the hypercoagulability (Virchow’s) triad.
• There are significant risks associated with anticoagulant therapy, so it should be undertaken with care.
INTRODUCTION
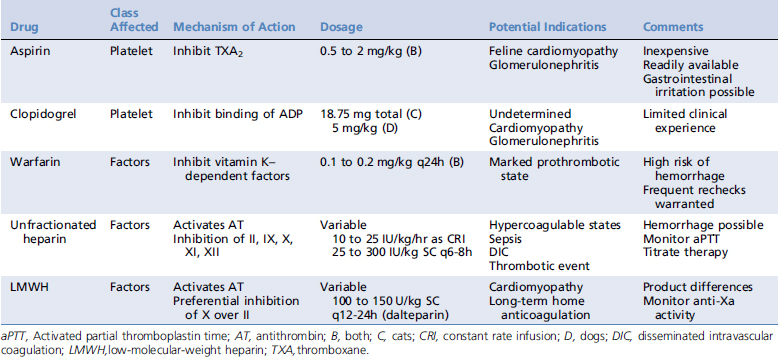
In light of differences in the pathogenesis of thrombi, anticoagulant strategies include altering platelet function or affecting clotting factor activity. For ease of classification, anticoagulant medications are commonly divided into (1) antiplatelet drugs, (2) oral anticoagulants, and (3) parenteral anticoagulants. Recommended dosages of these agents are listed in Table 187-1.