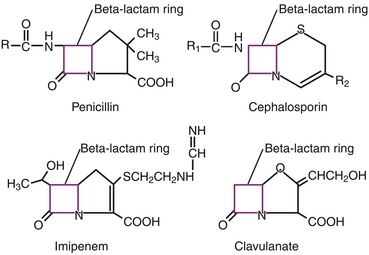
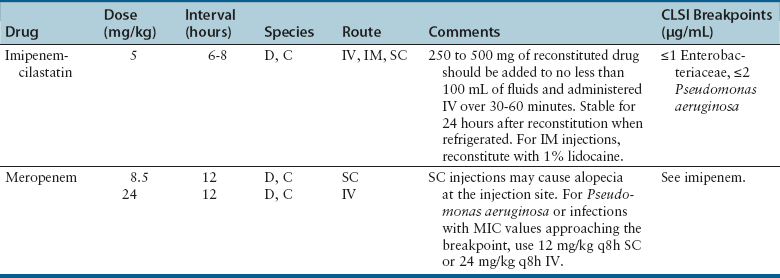
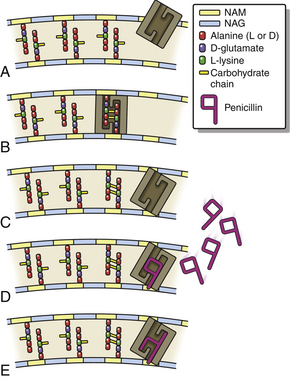
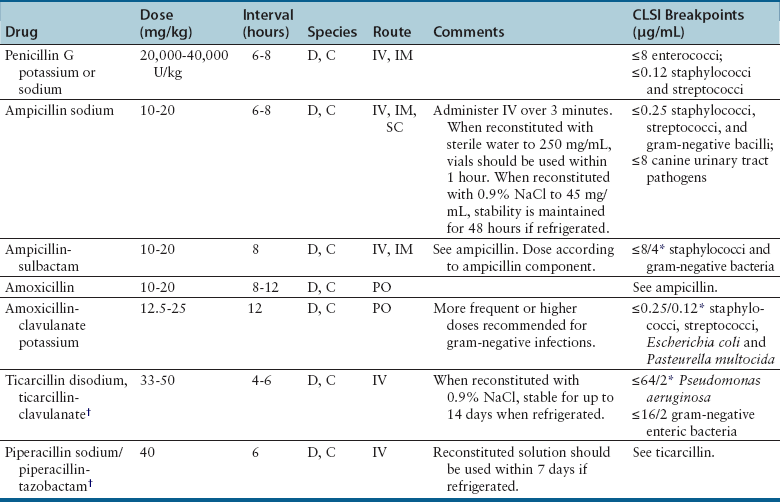
Chapter 8 • An understanding of the mechanisms of action, spectrum of activity, pharmacokinetics, pharmacodynamics, and adverse effects of antibacterial drugs facilitates optimal treatment of bacterial infections while minimizing the chance for (1) selection of resistant bacteria, (2) adverse reactions, and (3) drug interactions. • The importance of appropriate administration of antibiotics, compliance issues, and potential adverse effects of the drug chosen should be discussed with the pet owner when antibiotics are prescribed. This chapter reviews the classification, mechanisms of action, spectrum of activity, resistance mechanisms, tissue penetration, clinical use, and adverse effects of the major classes of antibiotics used to treat dogs and cats. Antimicrobials have been grouped together alphabetically according to their mechanism of action (cell wall synthesis, nucleic acid, and then protein synthesis inhibitors). Tables are dispersed throughout the text with drug dosages for each group of antibiotics. The reader is encouraged to review the accompanying text when using the tables. Not all antibiotics are reviewed here. Antibiotics that have specific uses, such as certain antimycobacterial drugs and nitrofurantoin, are reviewed in the relevant chapters of this book. Chapter 6 provides an overview of the basic principles of antimicrobial treatment. β-Lactam antibiotics have a β-lactam ring in their molecular structure (Figure 8-1). They include penicillins, cephalosporins, monobactams, and carbapenems. They are bactericidal antibiotics (see Chapter 6) that bind covalently to and inhibit penicillin binding proteins (PBPs). These PBPs are needed to catalyze the cross-linking (or transpeptidation) of the peptidoglycan layer of bacterial cell walls, which is continuously remodeled by bacteria (Figure 8-2). When PBPs are inactivated by β-lactam antibiotics, bacterial enzymes that hydrolyze the peptidoglycan cross-links during cell wall remodeling continue to function, which breaks down the cell wall further. The accumulation of peptidoglycan precursors also triggers activation of cell wall hydrolases, with further digestion of intact peptidoglycan. The end result is bacterial rupture. FIGURE 8-2 Interference of peptidoglycan production by β-lactam antibiotics. The bacterial cell wall consists of N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM) subunits. A, The penicillin binding protein (PBP, brown squares) binds peptide side chains and forms a cross-link (B). C, The PBP dissociates from the wall once the cross-link has been formed. D, Penicillins enter the active site of the PBP. E, The β-lactam ring of the penicillin is irreversibly opened when it reacts with the PBP, which permanently blocks the active site. The peptidoglycan layer of gram-positive bacteria is 50 to 100 times thicker than that of gram-negative bacteria and is highly cross-linked, which maintains the structural integrity of gram-positive bacteria.1 Therefore, compared to gram-negative bacteria, gram-positive bacteria are more easily inactivated by β-lactam antibiotics. Bacteria possess multiple PBPs, which are assigned numbers based on their molecular weight. PBPs vary in their affinities for different β-lactam antibiotics, which in part explains the difference in spectrum of activity and bactericidal action of β-lactam antibiotics. For example, inhibition of PBP1a and PBP1b leads to cell lysis, whereas inhibition of PBP2 results in rounded cells called spheroblasts. Drugs that produce rapid lysis (e.g., carbapenems) are the most bactericidal and have highest affinity for PBP1. Resistance to β-lactam antibiotics is now widespread and results primarily from β-lactamase production.2 It can also result from production of altered PBPs (such as PBP2a) and, among gram-negative bacteria, exclusion of drugs that normally diffuse through porins to their site of action. Gram-negative β-lactamases are strategically located just beneath the outer lipopolysaccharide layer, which acts as the barrier to drug penetration. Gram-positive bacteria secrete β-lactamases into their immediate surroundings. There are many different β-lactamase enzymes that vary in their specificity for β-lactam drugs.3 β-Lactam antibiotics have a short half-life and exhibit time-dependent pharmacodynamics (see Chapter 6). Drug concentrations should be maintained above the minimum inhibitory concentration (T > MIC) for at least 50% of the dosing interval. For penicillins and carbapenems, T > MIC can be less than 50%, but aiming for a target of 50% ensures that most patients will have adequate exposure. To maintain this target, some β-lactam antibiotics with short half-lives require frequent administration or slow infusion. For other drugs, a long half-life prolongs the T > MIC to allow for infrequent administration. For example, cefpodoxime proxetil has a half-life longer than those of other oral cephalosporins, so it can be administered only once per day. Cefovecin has an extremely long half-life in dogs and cats and can be effective for some infections when administered at 14-day intervals. For other cephalosporins (e.g., injectable drugs with short half-lives such as cefotaxime), three- to four-times-daily dosing may be required for treatment of gram-negative bacterial infections. Classification, Spectrum of Activity, and Resistance Mechanisms Penicillins are derived from Penicillium molds. They are classified as naturally occurring penicillins such as penicillin G (benzyl penicillin), the semisynthetic aminopenicillins such as ampicillin and amoxicillin, penicillinase-resistant penicillins, and extended-spectrum penicillins. Table 8-1 shows the classification of the penicillins and their respective spectra of activity. TABLE 8-1 Classification and Spectrum of Activity of Penicillin Antibiotics Penicillinase-resistant penicillins (antistaphylococcal penicillins) such as methicillin have a structure that resists inactivation by β-lactamase enzymes. Methicillin is no longer used clinically. Drugs such as flucloxacillin and dicloxacillin have replaced the use of methicillin, because they can be administered orally and have fewer adverse effects. These drugs are highly protein-bound, which delays their elimination and maintains plasma drug concentrations, but tissue penetration is sufficient for treatment of skin and soft-tissue infections. Although dosages of penicillinase-resistant penicillins have been published for dogs and cats,4 they are rarely used because other alternatives exist such as the combination of amoxicillin and the β-lactamase inhibitor clavulanic acid. Methicillin and oxacillin are used in the laboratory for susceptibility testing. Resistance to methicillin results from bacterial production of an altered penicillin-binding protein (PBP2a), which leads to resistance to all β-lactam antibiotics.5 Penicillin dosages for dogs and cats are listed in Table 8-2. Penicillins are distributed to the extracellular fluid of most tissues. Because of low penetration across the blood-brain barrier and blood-ocular barrier, high doses are needed in order to attain effective levels in these tissues. Penicillins undergo rapid renal elimination, and active drug is concentrated in the urine, so they are useful for treatment of bacterial urinary tract infections (UTIs). Liver impairment has little effect on the pharmacokinetics of penicillins. Increasing the length of the dose interval is important in order to minimize toxicity in animals with renal failure. TABLE 8-2 Suggested Penicillin Dosages for Small Animals C, Cats; CLSI, Clinical and Laboratory Standards Institute; D, dogs. ∗The “/” distinguishes the ticarcillin from clavulanic acid concentrations. †It is recommended that the use of these drugs be reserved for life-threatening infections that are susceptible to these drugs but resistant to other drugs, on the basis of culture and susceptibility testing. Penicillins are generally well tolerated by dogs and cats. The most common adverse effects in dogs and cats are vomiting, diarrhea, and inappetence. These are more likely to occur with amoxicillin when clavulanic acid is administered concurrently. Hypersensitivity reactions that include fever, cutaneous reactions, immune-mediated cytopenias, and anaphylaxis are possible, especially in dogs, but are less common than in human patients. The use of penicillins should be avoided in animals with a history of such reactions. Rapid intravenous infusion or administration of high doses of penicillins to animals with coexisting renal failure is rarely followed by development of neurologic signs, such as seizures. Pain or tissue reactions can occur when penicillins are administered intramuscularly or subcutaneously. High doses of extended-spectrum penicillins such as ticarcillin have been associated with platelet function abnormalities and significant bleeding in human patients.6 Classification and Spectrum of Activity The cephalosporins were originally derived from Acremonium spp. (previously known as Cephalosporium spp.). They have been broadly grouped into first-, second-, third-, and fourth-generation cephalosporins based on their spectrum of activity. As the generation increases, there is an increase in gram-negative spectrum, and fourth-generation drugs have truly broad-spectrum activity (Table 8-3). An exception to this pattern is the newest addition to the group, ceftaroline. It has activity against methicillin-resistant staphylococci as a result of an affinity for PBP2a and has been considered a “fifth-generation” cephalosporin. Cephalosporins are resistant to staphylococcal β-lactamase, and the extended-spectrum cephalosporins (primarily the third-generation drugs) are resistant to many gram-negative β-lactamases. However, extended-spectrum β-lactamase (ESBL) enzymes can hydrolyze even third-generation cephalosporins and present an important therapeutic challenge. TABLE 8-3 Classification and Spectrum of Activity of Cephalosporin Antibiotics Cephalosporin dosages for cats and dogs are listed in Table 8-4. First-generation cephalosporins such as cephalexin are moderately absorbed orally and distributed into extracellular fluids. In general, they have poor penetration of blood-brain barrier, even in the face of inflammation. Most are excreted unchanged in the urine, and so are useful for treatment of UTIs. Cefazolin has greater activity against a wider range of bacterial isolates than the oral first-generation cephalosporins (e.g., cephalexin and cefadroxil).7 Cefazolin has been a popular injectable antibiotic for perioperative use in small animals. This perioperative use of cefazolin is with the intention to prevent postsurgical infections, particularly in orthopedic surgery (see Chapter 85). TABLE 8-4 Suggested Cephalosporin Dosages for Small Animals C, Cats; CLSI, Clinical and Laboratory Standards Institute; CNS, central nervous system; D, dogs. ∗Dose not established for cats. †It is recommended that the use of most second-, third-, and fourth-generation cephalosporins be reserved for serious infections that are susceptible to those drugs but resistant to other reasonable alternatives on the basis of culture and susceptibility testing. Use of second-, third-, and fourth-generation cephalosporins may be indicated when there is resistance to first-generation cephalosporins and there are no other reasonable alternatives available. Three third-generation cephalosporins are approved by the U.S. Food and Drug Administration (FDA) for use in small animals: ceftiofur, cefpodoxime proxetil, and cefovecin. These agents are approved for treatment of skin and soft-tissue infections (cefpodoxime proxetil and cefovecin) and UTIs in dogs (ceftiofur). Although their use in small animal medicine has been controversial because of concerns that relate to selection for resistant bacteria, there is no evidence at this time that the use of these agents has been associated with increased resistance among isolates from dogs and cats. The injectable third-generation cephalosporins approved for people have greater in vitro activity than cefpodoxime or cefovecin and occasionally have been used in dogs and cats (e.g., cefotaxime, ceftazidime, cefoperazone). These drugs have a high degree of activity, but are expensive and must be administered frequently by injection. Distribution and excretion into urine and bile is variable. Some third-generation cephalosporins have a longer duration of activity than first- and second-generation drugs by virtue of long half-lives. Cefpodoxime proxetil is the most commonly used oral third-generation drug in small animals, approximately 50% of which is excreted in the urine in active form. Cefovecin is approved for use in dogs and cats in Europe and North America as a subcutaneous injection. The high protein binding and slow clearance result in a very long half-life in these species (approximately 7 days in cats and 5 days in dogs). Administration of cefovecin to cats results in a duration of activity of at least 14 days, and for at least 7 to 14 days in dogs (depending on the pathogen). Injections can be repeated in dogs at 14-day intervals if necessary. Adverse effects of cephalosporins in dogs and cats are similar to those of penicillins, with gastrointestinal signs and hypersensitivity reactions being most frequent. Although the incidence is low, cross-sensitivity can occur between penicillins and cephalosporins, so cephalosporins should be avoided or used with caution in animals that have a history of reactions to penicillin. Injectable cephalosporins can cause phlebitis and pain on injection. Cephalosporins have the potential to cause false-positive results on test strips that use copper reduction for urine glucose detection. Certain cephalosporins, such as cefotetan and ceftriaxone, may exacerbate bleeding tendencies due to vitamin K antagonism.8 Reversible bone marrow suppression has been reported in dogs given high doses of ceftiofur, cefonicid, and cefazedone long-term.9 Aztreonam is a synthetic β-lactam antibiotic that is resistant to some β-lactamases. It is effective against a wide range of gram-negative bacteria, including Pseudomonas aeruginosa, but not gram-positive or anaerobic bacteria. In human medicine, aztreonam is used to treat patients with serious gram-negative infections who are allergic to penicillins and cannot tolerate aminoglycosides.10 Dosages have not been established for dogs. Spectrum of Activity and Clinical Use Carbapenems are highly resistant to almost all bacterial β-lactamases and include imipenem, meropenem, ertapenem, and doripenem. They penetrate the outer membrane of gram-negative bacteria more effectively than many other β-lactam antibiotics and bind to a variety of PBPs, which leads to rapid lysis of a broad spectrum of bacteria.11 In contrast to other β-lactam antibiotics, carbapenems have a postantibiotic effect (see Chapter 6) similar to that of aminoglycosides and fluoroquinolones. Although effective against many gram-positive, gram-negative, and anaerobic bacterial infections, the use of carbapenems is reserved for treatment of serious, multiple drug-resistant (MDR) gram-negative infections, especially Escherichia coli, Klebsiella pneumoniae, and P. aeruginosa, that are resistant to other antibiotics. Methicillin-resistant staphylococci are resistant to carbapenems. Dosages for dogs and cats are listed in Table 8-5. The identification of carbapenemase production by some strains of gram-negative bacteria has raised great concern, because this confers resistance to a broad range of β-lactam antibiotics. Carbapenemase-producing K. pneumoniae (KPC) are resistant not only to all β-lactam antibiotics, but also to fluoroquinolones and aminoglycosides.12 K. pneumoniae that produce a plasmid-encoded metallo-β-lactamase enzyme (NDM-1) appeared in India in 2008 and have subsequently spread worldwide.13 The only options left for treatment of these infections are tigecycline and polymyxins, both of which have been associated with emergence of resistance during treatment.14 The potential for spread of K. pneumoniae isolates that are resistant to all commercially available antibiotics now exists. Carapenemase (NDM-1) production was recently detected in an E. coli isolate from a cat in the United States. Adverse effects of carbapenems are otherwise rare and include vomiting, nausea, and pain on injection. Neurologic signs, including tremors, nystagmus, and seizures, can occur following rapid infusion of imipenem-cilastatin or in animals with renal insufficiency. Imipenem must be administered slowly in intravenous fluids (see Table 8-5). Slow administration is not required for meropenem, which is more soluble. Subcutaneous injections of meropenem have produced alopecia at the injection site,4 but otherwise these injections have been very well tolerated and have eradicated infections associated with MDR gram-negative bacteria. Mechanism of Action and Clinical Use Vancomycin was discovered in the 1950s, but has not been frequently used in small animals because it is not absorbed orally, so IV infusion is required for treatment of systemic infections. When administered IV, it can be valuable for treatment of MDR gram-positive bacterial infections, such as methicillin-resistant staphylococcal infections, resistant enterococcal infections, and encrusting cystitis caused by Corynebacterium urealyticum. The last is an emerging infection of dogs, cats, and immunocompromised human patients, treatment of which requires surgical debridement of the bladder in conjunction with glycopeptide administration.15,16 Vancomycin is a valuable agent for treatment of methicillin-resistant staphylococci, because resistance among these organisms is extremely rare, and the bactericidal activity can be an advantage when treating immunocompromised patients. In human patients, oral vancomycin has been used to treat gastrointestinal infections caused by resistant Clostridium difficile. Vancomycin-resistant methicillin-resistant Staphylococcus aureus (MRSA) isolates are extremely rare in people and have only been described in specific geographic locations. Vancomycin-resistant Staphylococcus pseudintermedius isolates have not yet been described. Vancomycin-resistant enterococci (VRE) are well documented in human patients and can present a therapeutic challenge. Although currently rare, the existence of VRE has been reported in companion animals.17 Resistance to vancomycin results from bacterial alteration of the terminal amino acid to which vancomycin binds. In order to reduce inappropriate use of vancomycin, some laboratories do not report results for vancomycin susceptibility unless reporting is indicated on the basis of resistance in the remainder of the susceptibility panel. Teicoplanin has a longer half-life and is up to 100 times more lipophilic than vancomycin. It is not commercially available in the United States but is used in Europe. Vancomycin and teicoplanin appear to be equally efficacious in human patients.18 Vancomycin is not commonly used to treat small animals, and so adverse reactions have not been well documented. The most common reaction in human patients is the “red man syndrome” caused by histamine release after rapid infusion. This can be prevented through the use of antihistamines and slower infusion rates. Other adverse reactions are rare. Previously cited nephrotoxicity from vancomycin may be exaggerated because of its frequent co-administration with aminoglycosides. Current formulations are of better quality and purity and less likely to produce kidney injury. Vancomycin is given by IV infusion over 30 to 60 minutes through a central or peripheral catheter (Table 8-6). Trough plasma concentrations can be monitored and should be greater than 10 µg/mL for skin and soft tissue infections, and 10 to 20 µg/mL for more complicated infections. Intramuscular administration is painful and irritates tissues. TABLE 8-6 Suggested Vancomycin Dosages for Small Animals C, Cats; CLSI, Clinical and Laboratory Standards Institute; CRI, continuous-rate infusion; D, dogs. ∗The use of vancomycin should be reserved for gram-positive infections that are susceptible to vancomycin but resistant to all other reasonable alternatives, on the basis of culture and susceptibility testing. Resistance to fluoroquinolones results from DNA gyrase mutations, decreased bacterial permeability, and increased drug efflux.19 Susceptibility to one fluoroquinolone generally predicts susceptibility to others, with the exceptions of third-generation fluoroquinolones (such as pradofloxacin) and ciprofloxacin, which has higher in vitro activity against P. aeruginosa than other fluoroquinolones.20,21 Limited use of fluoroquinolones has been recommended by some human and veterinary medical authorities in order to minimize further development of resistance, but a clear association between prescription of fluoroquinolones and increased resistance has not been established in companion animals. Nevertheless, other “first line” drugs are often available for most infections, and the valuable fluoroquinolones can be reserved for cases that cannot tolerate other drugs, or when resistance to other drugs is more likely. A sufficiently high dose should be used to minimize selection of resistant isolates. Fluoroquinolones are most valuable to treat gram-negative infections, because there are often few oral medication alternatives for these infections. The fluoroquinolones are also effective for infections caused by staphylococci, but staphylococci tend to have higher MICs for fluoroquinolones than gram-negative bacteria. The activity of fluoroquinolones against anaerobes is variable. Pradofloxacin has the greatest activity, followed by marbofloxacin and enrofloxacin.22 Fluoroquinolones are concentration-dependent antibiotics, so they are best administered as a single high daily dose. Doses for fluoroquinolones are listed in Table 8-7. TABLE 8-7 Suggested Fluoroquinolone Dosages for Small Animals ∗The use of enrofloxacin to treat cats is controversial because of the potential for irreversible retinal toxicity and blindness. Do not exceed a dose of 5 mg/kg in cats. The use of alternative fluoroquinolones such as marbofloxacin, orbifloxacin, or pradofloxacin should be considered for cats. †Tablet formulation. The use of pradofloxacin at high doses in dogs has been associated with myelosuppression and is off-label in the United States, but it is approved for both dogs and cats outside the United States at a dose of 3 mg/kg (tablets). ‡Oral suspension for cats. The dose of the oral suspension for cats is higher (5-7.5 mg/kg) owing to reduced bioavailability. Fluoroquinolones are usually well absorbed after oral administration. Poor absorption occurs when they are complexed by divalent and trivalent cation-containing medications (e.g., antacids) and supplements (aluminum, calcium, iron, zinc). Use of these should be avoided if animals are receiving fluoroquinolones by the oral route. Oral absorption of the human drug ciprofloxacin is lower and more variable in dogs than in people, which necessitates administration of a higher dose. In one study, oral absorption of generic ciprofloxacin tablets in dogs was 58%, but the coefficient of variation was 45%.23 In cats, only 22% to 33% of orally administered ciprofloxacin is absorbed. Most fluoroquinolones are highly concentrated in the urine, so they are useful for treatment of UTIs. Enrofloxacin is metabolized to ciprofloxacin, which is subsequently excreted in the urine. Approximately half of the administered dose of marbofloxacin and orbifloxacin is excreted as unchanged drug in the urine. In contrast, difloxacin is excreted primarily in bile, and little drug enters the urine.24 Fluoroquinolones have variable protein binding, but this does not inhibit distribution to tissue fluids. Because of good lipophilicity, these drugs penetrate the prostate and respiratory secretions. They also penetrate the ear canal, but high doses may be required for treatment of bacteria with relatively high MIC values.25 Fluoroquinolones can attain high intracellular concentrations and can be used to treat infections caused by intracellular pathogens such as Mycoplasma spp. and some Mycobacterium spp. Fluoroquinolones can occasionally produce gastrointestinal signs in dogs and cats, such as decreased appetite and vomiting. Rapid IV administration can cause systemic hypotension, tachycardia, and cutaneous erythema, possibly as a result of histamine release.26 Neurologic signs, including tremors, ataxia, and seizures, can occur in dogs and cats treated with high doses of parenteral fluoroquinolones. Although not well understood, this is thought to result from fluoroquinolone interference with the binding of γ-aminobutyric acid to its receptors in the central nervous system (CNS).27 In general, fluoroquinolones should be used cautiously in animals prone to seizures. Enrofloxacin causes hallucinations in humans and so is not marketed or approved for treatment of humans. Cats treated with high doses of enrofloxacin (generally >5 mg/kg/day), especially parenteral enrofloxacin, have developed blindness resulting from acute retinal degeneration, manifested as bilateral mydriasis with tapetal hyperreflectivity.28,29 This results from a functional defect in a fluoroquinolone transport protein in the cat, with subsequent accumulation of photoreactive drug in the retina.30 In general, the blindness is irreversible, but if it is detected early and medication is discontinued, some resolution may occur. Orbifloxacin also can produce retinal injury in cats, but the dose needed for this reaction is much higher than doses used therapeutically. Studies of marbofloxacin (reported by the manufacturer) and pradofloxacin31 showed no evidence of retinal toxicity in cats, even with high doses, although there are reports to the FDA of blindness in cats associated with the use of marbofloxacin. Cats with renal insufficiency or those treated with medications that increase plasma fluoroquinolone concentrations may be more likely to develop this adverse effect. Because they inhibit proteoglycan synthesis and chelate magnesium, fluoroquinolones can cause cartilage and joint toxicity in young animals, although the clinical importance of this has been questioned.32,33 In general, prolonged (>7 days) use of fluoroquinolones during the period of rapid growth in dogs (from about 1 to 2 months of age to 28 weeks) should be avoided if possible. Cats are thought to be more resistant to development of cartilage toxicity. Pradofloxacin has been associated with myelosuppression in dogs at doses greater than 10 mg/kg. Fluoroquinolones inhibit some cytochrome P450 enzymes, which decreases metabolism of other drugs. This occurs with theophylline in dogs; therefore, the dose of theophylline should be adjusted if administered concurrently. Like cephalosporins, fluoroquinolones can cause false-positive results on test strips for urine glucose detection. Cutaneous photosensitization occurs in human patients but is rare in animals. Other adverse effects reported in humans, but not in dogs and cats, include cardiac arrhythmias, tendinitis and tendon rupture, altered glucose metabolism, and hepatic dysfunction.34
Antibacterial Drugs
Introduction
Inhibitors of Cell Wall Synthesis
Penicillins
Penicillin Group
Examples
Antibacterial Spectrum
Natural penicillins
Penicillin G
Gram-positive aerobic bacteria (especially streptococci) and anaerobes. Pasteurella multocida may also be susceptible.
Aminopenicillins
Ampicillin
Amoxicillin
Gram-positive aerobic bacteria (cocci and bacilli) and anaerobes; some susceptible gram-negative bacteria.
Penicillinase-resistant penicillins
Methicillin
Oxacillin
Cloxacillin
Dicloxacillin
Susceptible β-lactamase producing gram-positive aerobic bacteria. Less active against penicillinase-susceptible organisms than penicillin G.
Extended spectrum carboxypenicillins
Ticarcillin
Carbenicillin
Increased activity against susceptible gram-negative aerobes, which includes Pseudomonas aeruginosa, Proteus spp., Klebsiella, and anaerobes. Some reduction in gram-positive spectrum. Destroyed by β-lactamases.
Ureidopenicillins
Mezlocillin
Piperacillin
Susceptible gram-positive and gram-negative aerobes, which includes Pseudomonas aeruginosa. Some loss of anaerobic spectrum. Destroyed by β-lactamases.
Penicillin–β-lactamase inhibitor combinations
β-Lactamase inhibitors: clavulanic acid, sulbactam, tazobactam
Susceptible β-lactamase producing gram-positive and gram negative aerobic bacteria and anaerobes.
Clinical Use
Adverse Effects
Cephalosporins
Cephalosporin Generation
Examples
Antibacterial Spectrum
First
Cephalexin
Cefazolin
Cefadroxil
Methicillin-susceptible staphylococci and streptococci and aerobes; some activity against gram-negative aerobes (especially cefazolin). Unpredictable activity against anaerobes.
Second
Cefaclor
Cefotetan
Cefoxitin
Improved activity against gram-negative aerobes and anaerobes compared with first-generation drugs. Cefoxitin has greater stability against anaerobic β-lactamases (such as those produced by Bacteroides) than other second-generation drugs.
Third
Cefixime
Cefpodoxime
Ceftiofur
Cefotaxime
Ceftriaxone
Cefovecin
Ceftazidime
Activity primarily against gram-negative bacteria. Some drugs in this group have less activity against gram-positive bacteria and anaerobes.
Fourth
Cefepime
Gram-positive cocci, gram-negative bacilli, many β-lactamase-producing gram-negative bacteria. Reserve for life-threatening infections where no other alternative is available.
Clinical Use
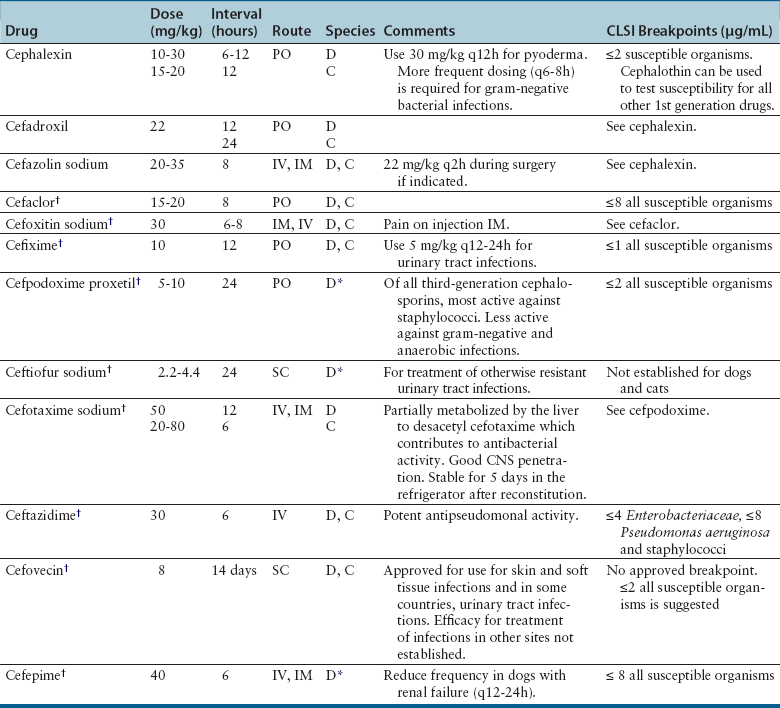
Adverse Effects
Monobactams (Aztreonam)
Carbapenems
Adverse Effects
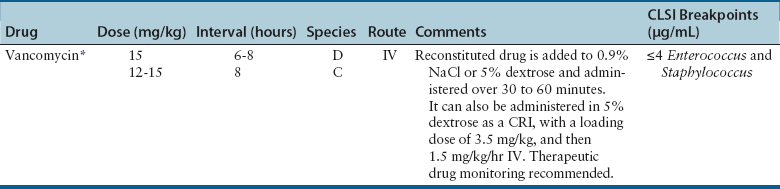
Glycopeptides
Adverse Effects
Nucleic Acid Inhibitors
Classification and Mechanism of Action
Clinical Use
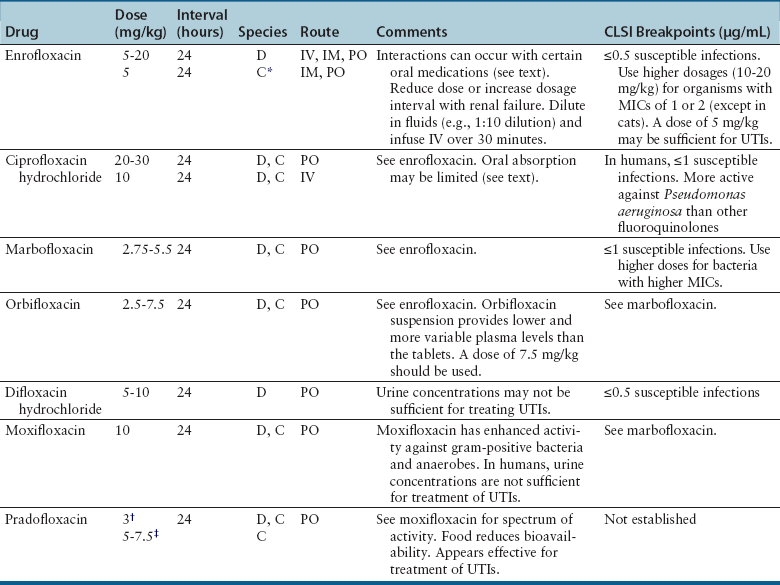
Adverse Effects
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Antibacterial Drugs
Only gold members can continue reading. Log In or Register to continue