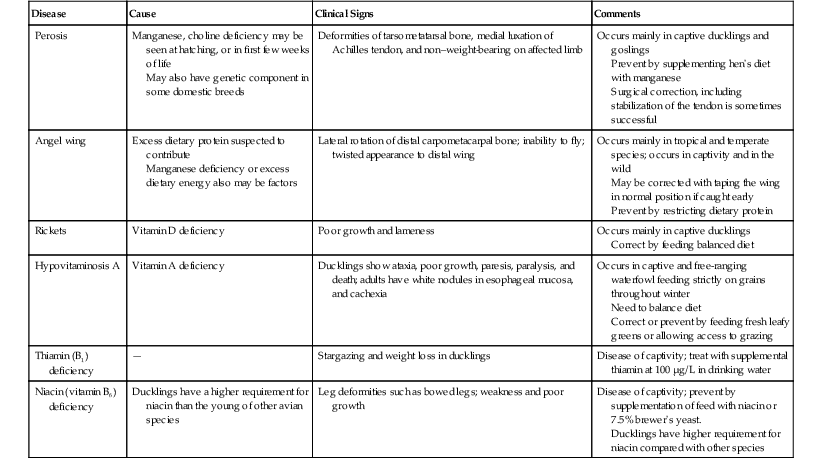
Kay A. Backues Until recently, the order Anseriformes (“waterfowl,”) has contained two families: (1) Anatidae, which includes the familiar ducks, geese, and swans and (2) Anhimidae, which includes the three species of very “un-duck-like” screamers native to South America. Recently, the Australian magpie goose, Anseranas semipalmata has been elevated to its own unique familial status on the basis of genetic studies, and the family Anseranatidae has increased the number of families under Anseriformes to three30,31 (Figure 16-1). The anatomic features of Anatidae and Anseranatidae are familiar to most veterinary practitioners—webbed feet, soft sensitive bills, relatively long necks with short legs, strong wings, and plumage that often includes heavy down feathers. The family of screamers is a bit more unusual. Aptly named for their loud raucous territorial calls, screamers lack the webbed feet and the soft, skin-covered bill of other Anseriformes. In addition, screamers possess complex systems of subcutaneous air sacs that may be contracted rapidly, an action that reportedly may produce a crackling sound!4 Additionally, they have extensive pneumatization of their bones, and even their phalanges may be pneumatized. They do not have distinct pterylae, or feather tracts; instead, their feathers are evenly distributed all over their bodies. Wild screamers are strong flyers and tend to form monogamous pairs, although large seasonal congregations occur, too. These birds are entirely herbivorous, and their tongues are keratinized and not fleshy as those of other Anseriformes species.4 Screamers are most often encountered in zoologic collections and have bred well in captivity. The Anatidae family contains the majority of species in this order, comprising approximately 150 species of ducks, geese, and swans.8 Referred to as waterfowl, they are important agricultural production animals, common zoo and private collection species, as well as frequently encountered wild bird species worldwide. Anatidae species range in size from a mere 275 grams (g) to 12 kilograms (kg) and include the heaviest birds capable of true flapping flight—the swans.19 Species such as the bar-headed goose (Anser indicus), which migrates over the Himalayas, represent some of the most remarkable vertebrate athletes in existence.19 The extensive seasonal migrations and the social nature of most species, both wild and domestic, expose these birds to many environments as well as to potential toxins and infectious diseases. In urban and suburban settings, the endemic wild species have adapted to human-dominated environments and mingle with domestic species. The opportunity for disease transmission, to the detriment of wild, captive, and domestic populations, has occurred repeatedly and continues to be a major concern for the agricultural industry and with regard to conservation efforts.10 Waterfowl, as their name implies, do best with constant access to clean water. Large, outdoor, planted enclosures with ponds, pools, or lakes are preferred. High stocking rates of waterfowl will lead to loss of turf and compaction of soil around lakes and ponds and promote unsanitary conditions associated with infectious diseases.3 For domestic and captive species, ensuring a place of refuge from predators (e.g., an island) and shelter from adverse weather is recommended. When artificial pools are provided, gently sloped sides to the pool are recommended to reduce abrasions to the plantar surface of the feet. Hospitalized waterfowl should have access to soft matted areas to cushion their feet and prevent abrasions that may lead to pododermatitis. The National Research Council (NRC) requirements for commercially raised ducks and geese are well established and provide a general reference for waterfowl diets.26 However, the overall protein and energy levels of commercially available waterfowl feeds are designed for maximum growth rates and should not constitute the sole diet of captive nonproduction animals. Herbivorous and granivorous, Anseriformes species do well when fed commercially available duck and/or poultry diets designed for maintenance as a base diet, with the addition of leafy greens and live insects, access to pasture and grazing, or both. The more carnivorous or piscivorous species require diets based on chopped fish or other specific dietary items. Table 16-117 lists selected nutritional deficiencies documented in Anseriformes. In the family Anseriformes, males have an intromittent copulatory organ attached to the ventral wall of the cloaca. This spiral-shaped phallus may be extruded manually from the first day of life by applying pressure to both sides of the cloaca; thus many species may be sexed at an early age.19 Sex determination by this method in adult nonsexually dimorphic Anseriformes species may be challenging, even with the use of sedation, with or without anesthesia, to relax the cloaca. Swans and geese form a lifetime pair-bond and may not form another bond if one member of the pair dies. Male ducks generally are not involved in egg incubation and may or may not breed with the same female in subsequent breeding seasons. Anseriformes young are precocial and leave the nest within hours of hatching, follow their mother, and are known for their strong imprinting instincts. Care of artificially brooded ducklings is similar to that of poultry chicks, by providing a round basin with central heat source. A round pen or basin of 0.5 to 1.0 square feet (ft2) per duckling is recommended for raising domestic ducklings, with a brooder temperature maintained at 85° F to 90° F for the first 2 to 3 weeks.6 An infra-red heat lamp positioned approximately 2 feet from the floor of the center of the pen provides warmth, and the ducklings may move closer to or away from it, as needed. Brooders should provide a soft but nonslip substrate to prevent spraddle or splay-leg in young ducklings, and the depth of substrate will depend on the species raised. Newspaper is not recommended unless it is shredded or pelleted to prevent it from becoming slippery; hay and straw beddings have been associated with aspergillosis.17 Most exotic species of ducks and geese that are similar to their domestic counterparts may be initially reared on a chick starter crumble that contains approximately 20% protein. The crumble may be wetted into a mash and the mash supplemented with grazing opportunities, whenever possible, to provide exercise as well as a diet of fresh greens. If grazing is initially not available, chopped fresh greens and live insects are both well accepted by neonatal waterfowl of most species. Food and water containers should have low sides to prevent very young ducklings from becoming wet. Neonatal ducklings are susceptible to chilling as their down is not waterproof. Chilled ducklings are poorly responsive and do not feed well.6 Ducklings may also become exhausted and drown if unable to exit the water container. Once they are no longer reliant on an external heat source, young waterfowl may be transitioned into larger holding facilities with pools or ponds that allow easy exit from the water. Predation from raptors, mammals, and reptilian carnivores, especially aquatic turtles in well-established ponds, are significant risks for young waterfowl that are housed outdoors. Restraint of captive waterfowl includes netting and herding for the catch-up, culminating in manual restraint. Some Anatidae species undergo a postbreeding molt, during which all the remiges, or flight feathers, are lost en masse in a very short period (hours to days) rendering them flightless for several weeks.19 In both wild and captive environments, this flightless period may be used opportunistically to herd and capture large numbers of waterfowl in some settings. When manually restraining waterfowl, handlers should take special precaution to avoid painful bites and scratches. Larger species may inflict strong and painful blows from their wings. Once captured, smaller species may be held single-handedly by restraining the animal with the wings folded or with fingers of one hand under each wing supporting the proximal humerus and the other hand supporting the bird’s abdomen. Supporting even small species only by the wings may lead to musculoskeletal injury, especially if the bird struggles. In one incident, a mallard-sized bird restrained only by the wings fractured a coracoid bone while struggling. Neurologic damage from such restraint has also been reported.17 In the case of larger species such as geese and swans, it is typical to hold the bird, wings folded and facing backward, under the arm of the handler (Figure 16-2). Large, relatively calm species may also be straddled on the ground to be restrained for oral administration of medications, gavage feeding, and minor procedures such as blood collection. Handlers must take special care not to put their body weight on the bird, but use their legs to keep the wings restrained and their hands to control the bird’s head and neck (Figure 16-3). Anesthesia should be administered to waterfowl for all but minor nonpainful procedures. Anesthesia or sedation is also often used as a method of safe restraint for nonpainful diagnostics such as radiography where manual restraint would be unsuccessful or contraindicated. Fasting for several hours is recommended prior to anesthesia. Prior to anesthesia, the crop should be palpated and its size and fullness assessed. If large amounts of food have been stored in the crop, non-emergency anesthetic procedures should be delayed. Inhalation anesthesia with isoflurane or sevoflurane is the most common in-hospital method for anesthetizing waterfowl. Induction is typically via a face mask. Intubation with a noncuffed endotracheal tube is recommended for all anesthetic procedures of more than 10- to 15-minute duration. Dose-dependent respiratory depression is seen in most anesthetized waterfowl, so occasional positive pressure ventilation is recommended. Waterfowl anesthetized with inhalant agents have been noted to present what is described as “rollercoastering” while anesthetized. The birds appear to be deeply anesthetized with slowing heart rate and respiratory rate but then suddenly lighten in their anesthetic depth, arouse, and move. This phenomenon may be associated with partial pressure of carbon dioxide (PCO2) chemoreceptors in the lung that influence respiratory drive.17,23 The addition of sedatives such as butorphanol and midazolam to the anesthesia regimen appears to lessen the degree of this fluctuating anesthetic depth in many waterfowl. Another complication in waterfowl is the development of thick mucus in the trachea or glottis during anesthesia. Mucus may completely plug the endotracheal tube or glottis during anesthesia or recovery and, if not removed, will lead to death of the bird. Thus, airway patency should be regularly checked during anesthetic episodes, the endotracheal tubes cleared, and the airway monitored until the bird has completely recovered. The most important piece of anesthetic equipment to ensure successful anesthesia of waterfowl is an attentive and experienced attendant whose sole responsibility should be the monitoring of anesthetic delivery, depth, and the patient’s vital signs during the entire anesthesia and recovery periods. An in depth review of anesthesia techniques and respiratory physiology of waterfowl as it pertains to anesthesia is published in detail elsewhere.23 Preparation of Anseriformes patients for surgery should take into account the individual species’ need for waterproofing and insulation. The feathers of Anseriformes species do not typically epilate easily, and most have small, tightly-packed contour and down feathers to provide waterproofing and insulation, respectively. The minimum amount of feathers should be plucked at the surgical site to provide a sterile field, and special care should be taken to avoid or decrease skin damage from plucking these tightly adherent feathers. Presurgically, only one feather or a few feathers at a time should be plucked and pulled in the direction that the feather lies to prevent tearing or bruising the skin. In addition, most waterfowl species do not have large apteria, and down feathers are evenly distributed over the entire body.19 Postsurgical care must take into account the loss of waterproofing and insulating properties caused by feather plucking. Following major surgical procedures, the bird may need to be withheld from access to water or swimming, depending on ambient and water temperatures, until significant feather regrowth occurs. The most common surgical procedure performed on captive waterfowl is pinioning. Done at 1 to 6 days of age, it is a relatively minor procedure that typically does not require plucking or general anesthesia, although a small amount of local anesthetic may be injected at the site prior to the procedure. The surgical site should be lightly prepped with a suitable topical disinfectant. The distal wing tip may then be clamped with a small hemostat, and the wing tip may be sharply removed distal to the clamp or directly amputated with a sharp pair of tissue scissors. Several minutes of moderate digital pressure at the amputation site is recommended to ensure hemostasis. It is common to apply a drop of skin glue at the site. The site of amputation is typically at the middle or proximal metaphyseal region of the metacarpal bones. The alula is often used as an external marker for the appropriate length at which to set the clamp and cut through the soft, cartilaginous major and minor metacarpal bones, but the length of the pinioned wing should be individualized for the species and the collection’s preferences. Neonatal pinioning has few adverse effects, whereas pinioning an adult bird is a major surgical procedure that requires careful tissue handling, attention to hemostasis, postoperative bandaging for at least 1 to 3 days, and postoperative pain relief. Adult pinioned birds are highly prone to repeated trauma to the pinioned site and often develop large fibromatous nodules that may be unaesthetic and prone to hemorrhage. The options of surgical feather follicle extirpation or laser ablation have been investigated as an alternative to the surgical pinioning of adult birds.20,29 In a study of two species of Anseriformes, the primary feathers were cut, a diode laser tip placed into the cut calamus of the treated primary flight feathers, and the follicle ablated with a total dose of 20 Joules of energy.29 Postoperatively, the treated wings showed swelling, edema, ulceration, and serosanguineous oozing. These side effects were deemed minor and completely resolved in all treated birds within 12 weeks. The laser treatment resulted in successful prevention of feather regrowth in 65% of treated follicles.29 The options of surgical follicle extirpation or nonsurgical follicle destruction in lieu of surgical pinioning should be considered for limiting flight in adult Anseriformes and warrant further study. For further information, see Chapter 65. The use of analgesics is necessary to provide humane care for painful injuries and procedures. Many publications have shown that different classifications of analgesics such as nonsteroidal anti-inflammatory drugs (NSAIDs) and opiates may have measureable effects in birds.25 However, the proof of analgesic efficacy, safety, proper dosage, and duration of analgesia for these drugs in many avian species, including Anseriformes, needs further study. Existing data demonstrating that analgesics administered to waterfowl do have some benefit via inhalant-anesthetic sparing effect are corroborated by the author’s experience.23,25 Both NSAIDs and opiates have been tested at various dosages in birds and some waterfowl species with varying results.2, 25 However, NSAIDs such as flunixin meglumine and ketoprofen have been shown to have side effects in many species of birds, including waterfowl. Flunixin meglumine was shown to reduce the production of cyclooxygenase (COX) enzyme-2, COX-2, but its use was associated with muscle necrosis at the site of injection in mallard ducks.21 Ketoprofen was associated with mortality in one study of eider ducks but has been used in studies of other waterfowl.24 Use of analgesics for painful procedures or injuries is considered part of humane care and best practice in modern veterinary medicine but is not without risk because of the lack of data on safety and efficacy in individual species. The extreme sensitivity of some Old World vultures to the NSAID diclofenac is an example of the variability of safety in different species to different drugs.27 The selection of an analgesic for use in any species should be based on a combination of the practitioner’s experience and a review of studies that show, at a minimum, the safe use of the selected drug in a particular species or a closely related species. The majority of diagnostic procedures for Anseriformes do not differ from those performed on other avian species. The long neck of many waterfowl species makes jugular venipuncture the preferred site for blood collection; other sites include the medial metatarsal or brachial veins. As in other birds, the right jugular is larger than the left and is the preferred site for obtaining larger quantities of blood. The venipuncturist holds the bird’s head and palpates the vein or at least the jugular groove cranial to the epaxial musculature and lateral to the trachea. This procedure works best if the neck is extended fully and the bird is adequately restrained. The medial metatarsal vein is found on the medial side of the tarsometatarsus; as in other birds, the site is an alternative for obtaining small quantities of blood. The brachial vein courses across the wing near the elbow and is easily found on most waterfowl species, but because of the limited soft tissue around the vein, venipuncture from this site is more likely to result in hematoma formation. Once obtained, blood may be placed in heparinized blood tubes or microtainers for a complete blood cell count or chemistry panel analysis. Some hematologic and plasma biochemistry reference ranges for selected Anseriformes species may be found in reference 5.
Anseriformes
Taxonomy and Biology
Unique Anatomy
Housing Requirements
Feeding
Reproduction and Neonatal Care
Restraint
Surgery and Anesthesia
Analgesia
Diagnostics
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree