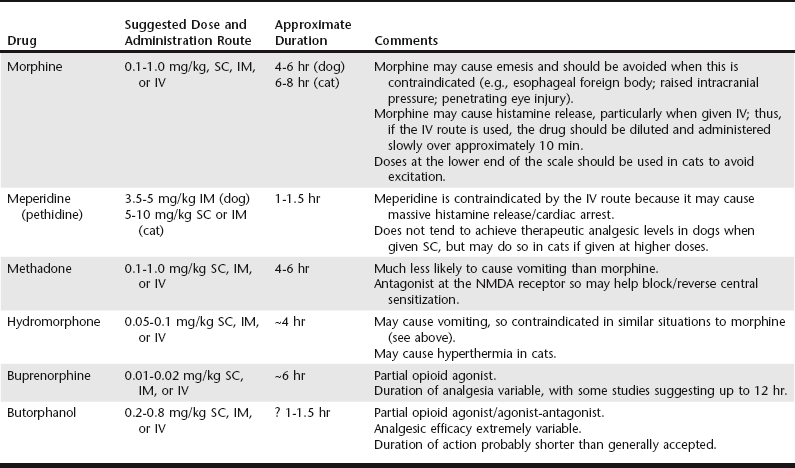
Chapter 13 Hypovolemia may be present as a result of dehydration (e.g., protracted vomiting with intestinal obstruction) or blood loss. Virtually all sedative and anesthetic agents cause a degree of CV depression, so the use of these drugs in patients with depleted circulatory volume can result in a severe drop in arterial blood pressure (BP). Consequently, restoration of the circulating blood volume should be undertaken prior to any form of chemical restraint, although there may be an argument for “delayed resuscitation” in those animals with internal hemorrhage (see Chapter 1). Cardiac arrhythmias are common in critical care patients. Although these may result from underlying cardiac disease, a number of extracardiac factors also may be responsible (e.g., hypoxemia, hypercapnia, electrolyte disorders). Regardless of etiology, the aim should be to restore normal cardiac rhythm before induction of anesthesia through treatment of underlying pathology, correction of exacerbating factors (e.g., hypokalemia), and judicious use (when necessary) of appropriate antiarrhythmic drugs (see Chapters 171 and 172). However, even with these measures, restoration of normal sinus rhythm may not always be possible, and consideration needs to be given to selection of an anesthetic technique that will not further exacerbate the arrhythmia. A variety of opioids (see Chapter 12) may be used in combination with the benzodiazepines, and the choice depends to a large extent on the procedure that the animal is undergoing. If there is preexisting moderate to severe pain, or if such pain is anticipated following the procedure, a full mu-agonist such as oxymorphone, hydromorphone, morphine, or methadone would be most appropriate because these can be titrated to the degree of pain. Partial agonists such as butorphanol or buprenorphine may be suitable alternatives but are less efficacious analgesics and should be limited to mild to moderate pain only. The full opioid agonists can also be antagonized (most commonly by naloxone) if the situation suddenly deteriorates, although this is uncommon if excessive doses are avoided. Table 13-1 lists suggested doses for each agent. TABLE 13-1 Opioids Commonly Used for Sedation/Premedication of Critically Ill Animals with CV Disease IM, Intramuscularly; IV, intravenously; NMDA, n-Methyl-D-Aspartic Acid; SC, subcutaneously. There is huge variability between individual patients in terms of dose requirements and analgesic response, and the doses/durations in Table 13-1 are intended only as a general guide.
Anesthesia for the Critical Care Patient
Anesthesia for Patients with Cardiovascular Dysfunction
Specific Patient Groups
Hypovolemic Patients
Patients with Cardiac Arrhythmias
Premedication/Sedation of Patients with CV Dysfunction
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


