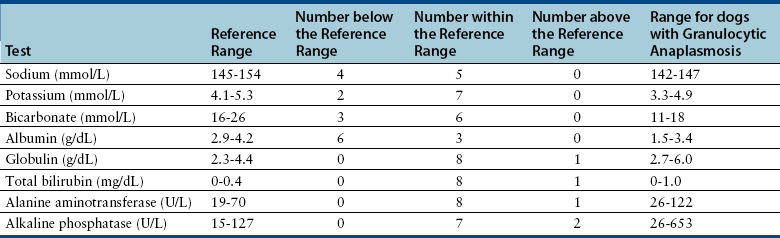
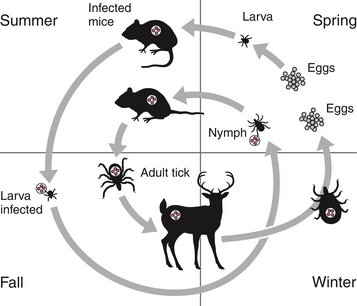
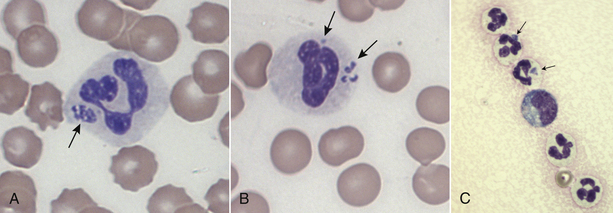
Chapter 29 The geographic distribution of the disease follows that of the tick vectors (Figure 29-1). In North America, the tick vectors of A. phagocytophilum are Ixodes scapularis in the northeastern and upper midwestern states, and Ixodes pacificus in the West. In Europe, the primary vector is Ixodes ricinus, and the disease has been described in dogs throughout continental Europe and in the UK. Ixodes persulcatus and Dermacentor silvarum ticks transmit the organism in Asia and Russia. Other Ixodes spp. ticks have also been implicated in transmission. Evidence of A. phagocytophilum infection has been found in dogs from Brazil and Tunisia, and a closely related organism was found in dogs from South Africa.4–6 In humans, rare reports exist of direct transmission that followed close contact with blood or respiratory secretions, transplacental spread, or transmission through blood transfusion. FIGURE 29-1 Distribution of Ixodes scapularis and Ixodes pacificus, the vectors of Anaplasma phagocytophilum, in the United States. The distribution of granulocytic anaplasmosis follows that of the tick vectors. Many seroprevalence studies of dogs have been reported worldwide.7 The seroprevalence varies with geographic location and whether the dogs studied were sick or healthy. The prevalence of positive antibody titers in dogs from some regions of Europe and North America exceeds 50%. A study that used a commercially available ELISA assay to determine the prevalence of seroreactivity to A. phagocytophilum in more than 400,000 dogs from the United States revealed wide variation in seroprevalences. Some counties in the upper Midwest and northeastern United States had seroprevalences that exceeded 40%, although the overall seroprevalence in these regions were 6.7% and 5.5%, respectively.8 Serologic cross-reactivity with A. platys may also influence these data. The seasonal pattern of disease reflects times of peak nymphal and adult tick activities, as well as periods when humans and their dogs are active outdoors. In the western United States, A. phagocytophilum infection occurs most frequently in dogs between April and July, when nymphal ticks are abundant. Some infections occur in October, during early questing of adult ticks. In Minnesota and Wisconsin, most cases are diagnosed in late spring (May, June) and fall (October, November).9–11 In a study from Berlin, most cases occurred between April and September.12 The median age of clinically affected dogs is 6 to 8 years (range, 6 months to 14 years).9,10,12–14 In the upper Midwest of the United States, a bimodal age distribution has been recognized, with 25% of dogs 1 year of age or less, and 50% of dogs at least 8 years of age.10 Affected cats in the northeastern United States have ranged from 4 months to 13 years of age (mean, 3.7 years).15 Although no breed predispositions are recognized, in one study, golden retrievers comprised almost half of affected dogs; this may reflect the popularity of these dogs for outdoor activities.14 Infection with other tick-borne pathogens is a risk factor for A. phagocytophilum infection. Co-infection with Borrelia burgdorferi, the cause of Lyme disease, is common because B. burgdorferi is transmitted by the same Ixodes tick species.8,10,11 In northern California, dogs seroreacted to A. phagocytophilum were 18 times more likely to be seropositive for Bartonella vinsonii subspecies berkhoffii than dogs that were seronegative.16 A. phagocytophilum is transmitted transstadially within the tick (i.e., from larva to nymph to adult), and not transovarially (from adult to egg). Dogs and cats thus become infected after exposure to infected nymphs or adult ticks, which acquire infection when they feed on wild animal reservoir hosts as larvae or nymphs (Figure 29-2). Ticks must attach for 36 to 48 hours for transmission to occur. Once A. phagocytophilum enters the bloodstream, it attaches to the sialylated ligands on the surface of neutrophils, such as P-selectin glycoprotein ligand-1.7 The organism then enters the neutrophil through caveolae-mediated endocytosis. A. phagocytophilum survives in the harsh neutrophil environment through dysregulation of neutrophil function and by bypassing phagolysosomal pathways. It inhibits neutrophil superoxide production and can reduce neutrophil motility and phagocytosis. A. phagocytophilum also reduces neutrophil adherence to endothelium and inhibits neutrophil transmigration into tissues, possibly through downregulation of selectin molecule expression.7 This may promote its survival in peripheral blood. FIGURE 29-2 Life cycle of Ixodes scapularis ticks and Anaplasma phagocytophilum infection. Uninfected larvae (top right) hatch in the late spring and acquire infection from small rodents in the summer. They then over-winter (often in protected mouse burrows) and then molt into nymphs the following spring. The nymphs feed in the late spring or early summer on a variety of animal species including rodents, humans, deer, and dogs. Nymphs molt into adults in the late summer to fall, which subsequently feed on large mammals such as deer, where they mate and drop off. The females then lay eggs and die. Dogs, cats and humans become infected by nymphs or adult ticks. Normally, neutrophils circulate for 10 to 12 hours before they enter tissues and undergo death through apoptosis. A. phagocytophilum delays neutrophil apoptosis, which allows it to survive longer periods of time within the neutrophil.7 A. phagocytophilum may also infect other cell types, such as bone marrow cells, endothelial cells, and megakaryocytes, although the importance of these cells in the pathogenesis of infection is not clear. The clinical signs and laboratory abnormalities that occur in dogs and cats with granulocytic anaplasmosis probably vary somewhat in different geographic locations as a result of local strain variation. The vast majority of dogs infected with A. phagocytophilum show no clinical signs. Some dogs and cats develop a self-limiting febrile illness, which in dogs occurs after an incubation period of 1 to 2 weeks. Lethargy occurs in almost all clinically affected cats and dogs. Fever and inappetence are also common findings. Lameness, reluctance to move, polydipsia, vomiting, diarrhea, and a soft cough can also occur. Generalized lymphadenopathy and splenomegaly develop as a result of reactive lymphoid hyperplasia and, in the spleen, concurrent extramedullary hematopoiesis.17 Uncommonly, hemorrhage, manifested as mucosal petechiae, melena, or epistaxis, has been reported in naturally infected dogs, but co-infections with other tick-borne pathogens may contribute to these signs in some dogs.9,12,13,18 Neurologic signs such as seizures, circling, cervical pain, and decreased placing reactions have been described in a few dogs from the upper Midwest with granulocytic anaplasmosis, but one dog with seizures had a history of idiopathic epilepsy.9,18 Neurologic signs and detection of the organism in the CSF have been uncommonly reported in humans.19 Infection with A. phagocytophilum results in mild to moderate thrombocytopenia, although other cytopenias may also occur (Table 29-1). The mechanism(s) of these hematologic abnormalities remain unclear. Anti-platelet antibodies occur in serum from humans and dogs with granulocytic anaplasmosis,12,20 and so immune-mediated mechanisms may contribute to thrombocytopenia. However, thrombocytopenia occurs in acute disease, before antibodies are noted, so other mechanisms may be important. The bone marrow of infected dogs shows megakaryocyte hyperplasia, so platelet destruction may be involved.21 TABLE 29-1 Hematologic Abnormalities in Nine Dogs with Granulocytic Anaplasmosis in Northern California∗ ∗Diagnosis was based on compatible clinical signs and either morulae within circulating neutrophils and/or a positive real-time PCR assay result for A. phagocytophilum. Impaired neutrophil function as a result of A. phagocytophilum infection may predispose to development of secondary opportunistic infections or influence the outcome of co-infections with other tick-borne pathogens such as B. burgdorferi. Although uncommon, opportunistic infections have been occasionally documented in humans and dogs with granulocytic anaplasmosis and are well in small ruminants.22 Infection with A. phagocytophilum may be self-limiting in dogs and cats, with minimal fatality or chronic disease manifestations. The extent to which A. phagocytophilum can persist in tissues and contribute to chronic disease manifestations in humans and dogs has been controversial, and may be dependent on the infecting strain and host immune response to infection. In one study, treatment of dogs that had been experimentally inoculated with A. phagocytophilum with prednisolone up to 6 months after infection was followed by the development of positive PCR results for the organism and, in some dogs, thrombocytopenia and reappearance of morulae on blood smears.23 One dog infected with a California strain of A. phagocytophilum was persistently PCR-positive through day 60 postinfection, the last time point evaluated.23 The most common findings on physical examination in dogs include lethargy, fever (up to 106.7°F [41.5°C]), dehydration, tachypnea, mild peripheral lymphadenopathy, and splenomegaly.7,10 Scleral injection may be noted. Lameness, reluctance to move, swollen joints, and pain on joint manipulation may also be detected in some dogs. Increased lung sounds, abdominal pain, epistaxis, petechial hemorrhages, and neurologic signs such as circling, cervical pain, and decreased placing reactions occur uncommonly. Physical examination findings reported in cats have been similar to those described in dogs, and include fever, lethargy, tachypnea, increased lung sounds, mild abdominal pain, hepatomegaly, splenomegaly, vomiting, ataxia, hyperesthesia, muscle and joint pain, lameness, conjunctivitis, and ocular discharge.15,24,25 Granulocytic anaplasmosis should be suspected in dogs and cats with acute febrile illness and thrombocytopenia that reside in endemic areas, regardless of tick exposure history. Diagnosis relies on detection of morulae within granulocytes, results of acute and convalescent serology, or molecular testing using PCR assays. The diagnostic criteria for confirmed human granulocytic anaplasmosis are clinical signs and laboratory findings suggestive of granulocytic anaplasmosis together with (1) detection of morulae within neutrophils combined with a single positive reciprocal antibody titer to A. phagocytophilum of at least 80; (2) a fourfold increase or decrease in the antibody titer within 4 weeks; (3) a positive PCR test result using specific A. phagocytophilum primers; or (4) isolation of A. phagocytophilum from blood.22 These criteria could also be applied to dogs. The use of multiple diagnostic modalities may be needed to confirm the diagnosis of granulocytic anaplasmosis in some dogs. Thrombocytopenia occurs in approximately 90% of dogs with granulocytic anaplasmosis.9,10,12,13 The platelet count in thrombocytopenic dogs may occasionally be as low as 5000 platelets/µL, although more often it is mild to moderately decreased (see Table 29-1).9,10,12–14 The majority of affected dogs are lymphopenic, but lymphocytosis can occur.9,12,13 Circulating reactive lymphocytes may be present. Anemia is common and typically mild and nonregenerative. Both neutrophilia and neutropenia occur, but most dogs have neutrophil counts that lie in the lower half of the reference range. Low numbers of band neutrophils, as well as mild neutrophil toxicity, are often present. Monocytopenia or monocytosis can occur.9,12,13 Cytologic examination of blood smears often reveals morulae within granulocytes (Figure 29-3). Morulae were detected in neutrophils from 36%, 56%, 67%, and 100% of dogs in four respective case series.9,12–14 Morulae appear as early as 4 days after experimental inoculation of dogs and persist for 4 to 8 days.17 The morulae are indistinguishable from those of Ehrlichia ewingii, so serology or PCR assays are needed to confirm A. phagocytophilum infection. FIGURE 29-3 Intracytoplasmic morula (A) and cocci (B) of Anaplasma phagocytophilum (arrows) within neutrophils of an 8-year-old male neutered golden retriever with granulocytic anaplasmosis; in (C), morulae are present in neutrophils within the synovial fluid of a 5-year-old curly-coated retriever that had polyarthritis. In contrast to dogs, thrombocytopenia appears uncommon in cats infected with A. phagocytophilum. None of 15 sick, PCR-positive cats from the northeastern United States were thrombocytopenic.15 The most common hematologic abnormality in cats has been lymphopenia. Morulae have been detected in some cats with granulocytic anaplasmosis. The most frequent serum biochemistry finding in dogs with granulocytic anaplasmosis is mild to moderate hypoalbuminemia (Table 29-2). Mild hyperglobulinemia, mild electrolyte abnormalities (hypokalemia, hyponatremia, and metabolic acidosis), and a mild increase in the activities of serum ALP and to a lesser extent ALT may occur.9,10,13,14 Urinalysis in dogs with granulocytic anaplasmosis may reveal isosthenuria, hyposthenuria, and proteinuria. Urine protein-to-creatinine ratios in two affected dogs from the United States were 1.5 and 2.2,10 but there is no evidence that A. phagocytophilum infection causes severe glomerulonephritis in dogs.
Anaplasmosis
Anaplasma phagocytophilum Infection
Clinical Features
Physical Examination Findings
Diagnosis
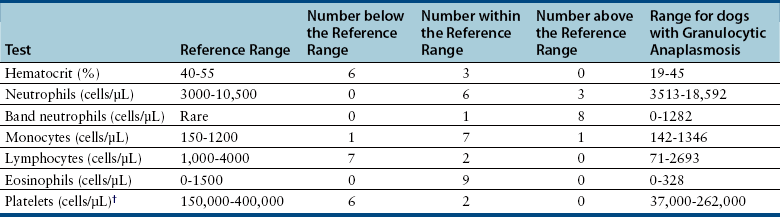
Laboratory Abnormalities
Serum Biochemical Tests
Urinalysis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Anaplasmosis
Only gold members can continue reading. Log In or Register to continue