CHAPTER 6 Analgesia
Recognition and management of feline pain are increasingly prominent in veterinary medicine. Given the 63.3 million veterinary visits made annually by an estimated 82.4 million cats owned in the United States,22,121 there is ample opportunity to include the assessment of pain as a routine component of a feline examination. Published surveys of analgesic use in cats over a 10-year span show a marked increase in the number of cats that now receive perioperative analgesics.34,55,65,66 Continuing professional education and review articles contribute to this phenomenon.55,65 Owners are also seeking and demanding appropriate pain management for their cats, both for surgical procedures and for chronic conditions such as degenerative joint disease.
However, there is room for improvement. Some cats continue to be denied analgesics for procedures such as castration, and very few cats receive analgesic agents in the postoperative period despite the fact that many procedures are likely to result in pain lasting several days.55 The perception by veterinarians that owners will not pay for analgesia is often given as a reason for the undertreatment of pain in cats. This assumption was not supported in a survey of owners in Finland, where 77% of respondents agreed that the cost of treating pain was not a concern.55,149 Between 78% and 98% of owners also agreed that treating their animal’s pain was somewhat to very important to them.149 The purpose of this chapter is to review the current state of knowledge on the recognition and treatment of acute pain in cats.
Pain Recognition and Assessment
The American Animal Hospital Association (AAHA) and the American Association of Feline Practitioners (AAFP) have published guidelines for incorporating pain management into veterinary practice.54 The first and pivotal step in the algorithm is assessing whether the animal is in pain. However, up to 42% of veterinarians consider their knowledge of pain assessment for both dogs and cats to be inadequate.59,163
The International Association for the Study of Pain (IASP) defines pain as “an unpleasant sensory or emotional experience associated with actual or potential tissue damage, or described in terms of such damage.”140 The emotional or affective aspects of pain are important but difficult to measure in nonverbal species. Assessment of pain in animals is based primarily on ethological quantification of behavior, but the wide range of feline “personalities” and variety of normal behaviors make this a challenge. Very subtle changes in behavior may indicate pain, and these can be easily overlooked by both owners and professional caregivers. Because the pain experience is unique to each individual, behaviors vary among cats, making standardization of assessment difficult. Behaviors related to fear and stress may be difficult to differentiate from those associated with pain. For example, one cat may be immobile and crouched in the back of a cage even when no painful procedure has been performed, whereas another cat displaying the same behavior may be in pain. For this reason understanding the individual patient’s normal behavior is imperative. Owners can provide valuable insight into their cats “normal” behavior and should be consulted.
A structured assessment tool is necessary both as a baseline and to monitor response to therapy. The components of such a tool must be user friendly, accurate, reliable, and time-efficient. Objective data, such as heart rate and respiratory rate, are easy to collect; however, there is poor correlation between this type of information and observed behaviors in animals after surgery.24 Blood pressure is a good objective indicator of pain in cats after ovariohysterectomy in a controlled environment, but in a clinical setting this tool is less reliable.131,132 The use of multiple indicators to assess feline discomfort is beneficial for compiling an overall picture. Compared to dogs, there is currently no robustly tested or validated composite acute pain scale for cats57,92; however, such pain scales are currently being developed. Preliminary data suggest that cats in pain show consistent changes in psychomotor behavior (e.g., comfort, activity, mental status), “miscellaneous behaviors,” and protective behaviors (e.g., response to surgical wound, abdominal or flank palpation) and that there is a correlation between pain and vocalization.13 Specific postures are also associated with abdominal pain.158
Although visual analog scales and numeric rating scales are technically easier to use compared with a composite pain scale, they are unidimensional, and interobserver variation is large; in one study variability was 36%.58 When a behavioral pain indicator is used, assigning a descriptor as well as a score assists in reproducibility and consistency of scoring.92 For example, under the heading “posture,” descriptors such as relaxed, hunched, and rigid could be added. The details of these descriptors and the weighting of scores are not fully worked out. Useful information in the hospital environment falls into several main categories, which are listed in Box 6-1.
A cat in pain shows little interest in interacting with caretakers, does not seek attention, has little interest in its surroundings, is more reclusive, has minimal interest in food, and may not groom itself normally (this may be exhibited either as lack of grooming or as excessive grooming, especially of the painful site). Cats in pain may urinate or defecate outside the litter box because it is too painful to move to or into it. The posture for a cat experiencing pain after abdominal surgery has been described as “half tucked up” or “crouching.”158
Figure 6-1 shows an example of a pain-scoring system based on posture. Figure 6-2 shows clinical examples that correspond to various places along the scale.
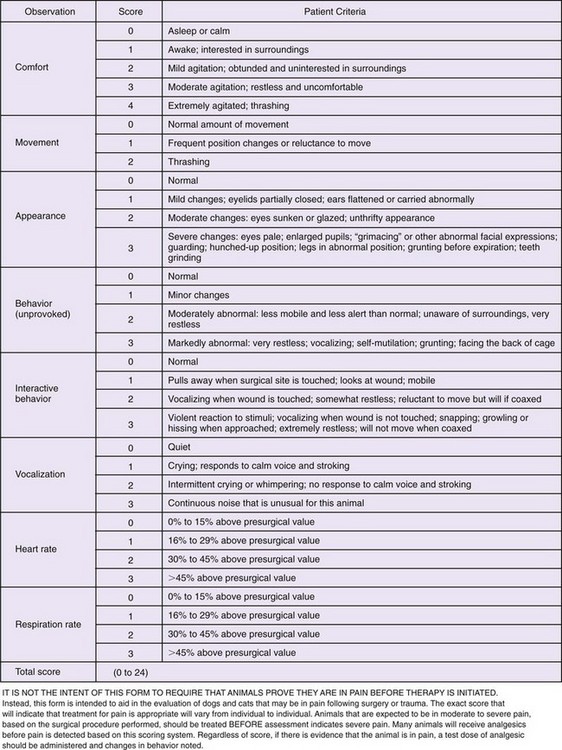
FIGURE 6-1 Example of a pain scoring system based on posture and behavior. Following surgery or trauma the goal is to maintain a score of <1.0. See Figure 6-2 for examples of scoring.
(From Hellyer PW, Gaynor JS: How I treat: acute postsurgical pain in dogs and cats, Comp Contin Educ 20:140-153, 1998.)
Facial expression has been used to assess pain in newborn infants118 and may also be useful in cats. Cats in pain often hold their heads low with their eyes half or fully shut and in a slanted position (see Figure 6-2).
A review of the relevant literature on the treatment of feline pain yields varying methods of assessment. In addition to the clinically applicable techniques previously described, several nociceptive threshold testing devices have been successfully adapted for use in cats. These are often used in a laboratory setting to screen putative analgesics for onset, intensity, and duration of antinociceptive actions before clinical testing is performed. In addition, different routes of administration can be compared. Thermal, mechanical, electrical, and visceral stimulation models have all been used in cats.10,31,32,35
As with all nonverbal species, cats depend on their owners to seek care and treatment for their ailments, including pain. Differences are present not just among individual cats but also among caretakers. Owners range from novices with a first pet to experienced owners who may care for multiple cats at any one time. This variability in exposure and experience can influence the owner’s understanding of a cat’s need for analgesia. For example, when owners who currently owned a cat were compared with owners who had no cat ownership experience, members of the former group were more likely to agree with a statement suggesting a similar pain experience between animals and humans,149 and this may lead to a higher value being placed on appropriate analgesia. In one survey of pet owners, 50% were concerned about postoperative pain,153,154 so it seems logical that these same owners would be receptive to learning about recognizing and alleviating pain.
Routes and Methods of Drug Administration
Analgesic drugs are given by many different routes, including parenteral (intravenous, intramuscular, subcutaneous), transdermal, topical, oral, transmucosal, and epidural. Careful thought regarding choice of route, both for ease of administration and efficacy, is necessary. For example, the same dose of hydromorphone has very different antinociceptive and side effects depending on whether it is given intravenously, intramuscularly, or subcutaneously.113
Sustained-Release Formulations
Long-acting formulations of drugs are advantageous in some cats. A sustained-release formulation of buprenorphine that is injected subcutaneously has been evaluated in cats.18
Constant-Rate Infusions
Opioids, ketamine, and alpha2-adrenergic agonists have all been administered to cats as constant-rate intravenous infusions (CRIs). The goal of a CRI is to achieve a steady-state concentration of the drug and avoid the peaks and troughs of intermittent treatment, thereby resulting in more consistent patient comfort. Selecting the loading and infusion rates to achieve a steady-state concentration requires species-specific pharmacokinetic data as well as plasma concentration–effect data that are not currently available for all analgesic drugs used in cats. However, pharmacokinetic and pharmacodynamic data are available for some opioids, such as fentanyl and remifentanil. Box 6-2 shows the steps for calculating a CRI.
BOX 6-2 Constant-Rate Infusion Setup
Step 2: Determine Dosage and Time Period for Analgesic Drug
Step 3: Programming a Syringe Pump for Delivery
Step 4: Using Maintenance Fluids for Delivery
• Determine total volume of fluids per kilogram per hour for administration (mL/kg/h).
• Determine the rate of administration of the constant-rate infusion in milligrams per kilogram per hour (mg/kg/h).
• Determine the total volume for infusion in mL.
• Divide the number of mg/kg/h by the mL/kg/h, with the end result in mg/mL.
• Multiple this number (mg/mL) by the total volume for infusion, resulting in the mg needed to add to the fluids.
• Divide this mg by the drug concentration in mg/mL.
• Add this volume of drug to the predetermined volume of fluids.
• Administer the fluid containing the drug at the rate selected.
Example of using maintenance fluids for delivery in a 5-kg cat receiving 3 µg/kg/h of 50 µg/mL fentanyl*
• Cat will get 2 mL/kg/h of maintenance fluids.
• The drug will be administered at a rate of 0.003 mg/kg/h (= to 3 µg/kg/h).
• We will put 6 h of fluids in a buretrol (60 mL).
• (0.003 mg/kg/h)/(2 mL/kg/h) = 0.0015 mg/h.
• (0.0015 mg/h)/(0.05 mg/mL) = 0.03 mL.
• Add 0.03 mL of fentanyl to 60 mL of selected fluid.
* Bear in mind increasing fluids will increase analgesic drug delivery, so desired changes in fluid administration and drug administration will occur concurrently. The advantage of a syringe pump is the analgesic drug administration is independent of fluid rate and varying the drug delivery has minimal consequences on fluid administration.
Transdermal Administration
It is easy to understand why a “hands-off” approach for delivering drugs to cats is attractive to caregivers insofar as it precludes the need for intramuscular or intravenous injections and may provide constant and long-term pain relief. Several drugs are available in the form of a transdermal patch, including lidocaine, and the opioids fentanyl and buprenorphine, all of which have been used in cats with varying success.71,82,96
Transdermal gels containing a wide variety of drugs have been touted by compounding pharmacies as effective in cats, and the simplicity of this technique is very attractive. Unfortunately, there is little scientific evidence to support that this method of administration results in effective uptake (see Chapter 4). Fentanyl formulated in a pluronic lecithin gel and applied to the shaved skin of the neck or to the pinnae of cats’ ears could not be detected in plasma,112 and this method is not recommended by these authors.
Topical Administration
Cream and gel preparations of local anesthetics are available and have been used in cats; they are helpful for placement of intravenous catheters.38,41,157
Oral Administration
Administering a drug by way of the oral route results in absorption from the gastrointestinal tract. In cats the most common analgesic drugs given by this route are the nonsteroidal antiinflammatory drugs (NSAIDs). Tramadol and gabapentin have good oral bioavailability,105,122 whereas first-pass metabolism of opioids limits their efficacy when given by this route. Palatability is a high priority with oral medications. The oral formulation of meloxicam, an NSAID, is highly palatable to cats,23,52,79 whereas tramadol is not (in the authors’ experience).
Oral Transmucosal Administration
Oral transmucosal, sometimes referred to as buccal, administration involves depositing the drug (usually liquid) onto the oral mucosa, where it is absorbed into the bloodstream, thereby avoiding first-pass metabolism. In cats the easiest approach is to deposit the drug in the cheek pouch or under the tongue. A variety of drugs can be administered this way in uncooperative patients, such as dexmedetomidine.128 Buprenorphine, an opioid that is discussed in detail later, has almost 100% bioavailability after oral transmucosal administration.109,111 Compared with other species that have a neutral oral pH, the more alkaline mouth of the cat may enhance absorption of this drug. Butorphanol has also been administered by the oral transmucosal route in cats, but it is not effective at maintaining the plasma concentrations that are achieved after intravenous dosing.161
Epidural
Administration of drugs into the epidural space can provide long-lasting analgesic benefit to the animal, with few systemic effects. The rate of complications for epidurals is low, although urinary retention was noted in 2 of 23 (8%) cats in one study.148 However, with appropriate nursing care (e.g., observing for urination, expressing the bladder after the procedure, placing a urinary catheter), this complication can be minimized. The spinal cord ends at L7-S1 in the cat, so careful needle placement and observation for the presence of cerebrospinal fluid is necessary to avoid administration of drugs into the subarachnoid space. If the subarachnoid space is entered, the drug volume is halved.73 Epidural catheters are available for use and have been used successfully in dogs139 but are not widely used in cats. The epidural space is approached after the cat has been anesthetized. This space is easily palpable with the cat in sternal recumbency, with the hindlimbs pulled forward. The animal is appropriately clipped and prepped; a sterile approach is vital to avoid bacterial contamination of the epidural space. Infected skin is an absolute contraindication to an epidural. The wings of the ilium are palpated bilaterally with the thumb and third finger while wearing sterile gloves, while the index finger palpates the lumbosacral space. Once the space is palpated, it is approached with a 1.5-inch 22-gauge spinal needle at a 45- to 90-degree angle to the skin, targeting midline of the animal and the center of the epidural space. Often, practice is necessary for proficient placement of a spinal needle. Blood in the spinal needle necessitates aborting the procedure.
Analgesic Drugs
The classic and most commonly used analgesic drugs include the opioids, NSAIDs, and local anesthetics. Historically, information on drug therapy in one species has been extrapolated to the cat without consideration of the ways in which the cat’s unique metabolism may alter both the pharmacokinetic profile and pharmacodynamic effect of the drug. Drug metabolism is discussed in Chapter 4, and this information should be kept in mind before using a drug in a cat for any reason, including pain management.
Opioids
Opioids are the cornerstone for the treatment of acute pain in many species, including the cat. The reasons for its popularity include efficacy, high margin of safety,9 and reversibility. Fortunately, there has been significant progress in dispelling the myth that opioid use in cats results in excitement, making their use inappropriate. So-called morphine mania was documented in the early literature, when doses as high as 20 mg/kg of morphine were used.138 It is now recognized that when clinically relevant doses of opioids are given, cats frequently display some euphoric responses, such as purring, rubbing, and kneading with the forepaws, and are easy to handle.109,112 Opioid administration is appropriate for trauma patients, cats undergoing surgery or invasive diagnostic procedures, and those with painful medical conditions (e.g., pancreatitis, cystitis).
Opioids are more effective when given before a painful procedure than after one because of their ability to decrease the development of central sensitization in response to surgical stimulation.82 This preemptive effect has been demonstrated in many species, including the dog77 and rat,83 and there is no reason to believe that it does not also occur in the cat. Therefore opioids should be incorporated whenever possible into premedication protocols for elective surgery. This does not mean that postoperative administration is unnecessary; continued assessment for comfort is essential after surgery and administration of analgesic drugs. Opioids or other analgesic agents may be necessary for several days, depending on the severity of the surgical procedure.
Side Effects of Opioids
Elevated body temperatures have been reported in cats given opioids between 1 and 5 hours after recovery from anesthesia.97,102 Hydromorphone at clinically recommended doses (0.05 to 0.1 mg/kg subcutaneously, intramuscularly, or intravenously) was associated with an increase in rectal temperature above 40o C (104o F) in 75% of cats in one study,97 and in one cat a rectal temperature of 42.5o C (108.5o F) was recorded. In another study most cats undergoing elective surgery that received hydromorphone, diazepam, and ketamine followed by isoflurane had postanesthetic temperatures that exceeded those recorded before anesthesia, with a peak rectal temperature of 41.6o C (107.0o F) reported in one cat.102 In a clinical setting, one study102 found that the lower a cat’s temperature was during anesthesia and surgery, the more severe the “rebound” hyperthermia.
In a laboratory study, hydromorphone at 0.1 mg/kg (administered intravenously) was associated with a significant increase in body temperature, whereas doses of 0.025 and 0.05 mg/kg were not. However, the lower doses were found to have minimal antinociceptive effects compared with the 0.1 mg/kg dose.159 Transdermal fentanyl patches resulted in higher rectal temperatures compared with butorphanol for cats undergoing onychectomy, although no temperature exceeded 40o C (104o F).40 Alfentanil infusions during anesthesia were also associated with increased rectal temperatures in cats.61
Recent laboratory studies in cats with implanted thermistors that did not undergo surgery showed that intramuscular administration of the opioids hydromorphone (0.05 to 0.2 mg/kg), morphine (0.5 mg/kg), buprenorphine (0.02 mg/kg), and butorphanol (0.2 mg/kg) alone or in combination with ketamine or isoflurane caused a mild to moderate increase in body temperature (≤40.1o C, 104.2o F), which lasted several hours but was self-limiting.103
After the end of anesthesia, body temperature is usually measured until the cat becomes normothermic, but as noted previously, it is prudent to monitor beyond that point and for 5 hours or longer after the end of anesthesia. Using warm air or circulating water blankets can prevent intraoperative hypothermia, which may in turn limit severe “rebound” hyperthermia. If profound hyperthermia develops, treatment includes active cooling or administration of naloxone (0.01 mg/kg intramuscularly, subcutaneously) or both.103
Some opioids, including morphine and hydromorphone, may cause retching, vomiting, and nausea characterized by salivation,110,113 especially when used alone and in pain-free cats (e.g., as a premedicant for an elective procedure). Vomiting and profuse salivation are common after subcutaneous administration of hydromorphone and appears distressing to the cats.113 Vomiting and retching are best prevented in cats with increased intraocular pressure, penetrating corneal foreign bodies, and elevated intracranial pressure. In many cases of foreign body ingestion (e.g., needles or linear objects), vomiting and retching can cause penetration of the gastrointestinal tract.
In dogs the administration of acepromazine reduces opioid-related vomiting151 and is potentially effective in cats. Clinically, vomiting occurs less commonly when opioids are combined with acepromazine than when they are used alone in cats. Maropitant, a neurokinin-1 antagonist, is highly effective against the emetic effects of xylazine in cats,56 but there are no reports of its use in conjunction with opioids. If vomiting is contraindicated in a feline patient but an opioid is required to provide pain relief, appropriate choices would include buprenorphine or methadone (intramuscular or intravenous) or fentanyl as a CRI.
In humans decreased intestinal motility is a common, unpleasant, and problematic side effect of opioid administration.120 In the authors’ experience, it is uncommon to see constipation in cats being treated for acute pain or when opioids are only used for a few days.
Potential Drug Interactions
With the increasing use of psychoactive drugs (selective serotonin reuptake inhibitors, tricyclic antidepressants, monoamine oxidase inhibitors and serotonin agonists; see Chapter 14) in veterinary medicine as part of a treatment regimen for behavior problems, there is a growing concern for the possibility of adverse drug interactions.9,90 Serotonin toxicity—which can range from mild signs such as salivation and diarrhea to severe signs such as myoclonus and hyperthermia resulting in death—can occur when two drugs that increase serotonin levels are co-administered. Meperidine (pethidine), fentanyl, remifentanil, pentazocine, and tramadol impair the reuptake of serotonin. Although not well documented in the veterinary literature, the addition of these analgesic agents to an established psychoactive drug protocol in humans has triggered serotonin toxicity.85 Before an analgesic plan is drawn up for a cat, it is essential to establish a list of all current medications. This includes any supplements or herbs the owner is administering; St. John’s wort (Hypericum perforatum), for instance, alters serotonin reuptake.
Specific Opioid Drugs
Current nomenclature of opioid receptors is based on molecular cloning. The three types of receptors—OP1, OP2, and OP3—were formerly known as delta (δ), kappa (κ), and mu (µ), respectively.123 Opioid drugs are traditionally classified by their actions on these receptors into agonists, partial agonists, agonist-antagonists, and antagonists. Suggested doses of commonly used drugs are given in Table 6-1.
TABLE 6-1 Suggested Dose Ranges and Route of Administration for Analgesic Drugs Commonly Used to Treat Acute Pain*
| Drug | Dose (Range) | Route of Administration |
|---|---|---|
| OPIOIDS | ||
| Butorphanol | 0.1-0.2 mg/kg | IV, IM |
| Buprenorphine | 0.02-0.03 mg/kg | IV, IM, OTM |
| Fentanyl | 2-10 µg/kg (bolus) | IV |
| 5-50 µg/kg/hour intraoperatively | IV | |
| 2-10 µg/kg/hour postoperatively or in trauma patients | IV | |
| Hydromorphone | 0.05-0.1 mg/kg | IV, IM |
| Oxymorphone | 0.05-0.1 mg/kg | IV, IM |
| Meperidine (pethidine) | 5 mg/kg | IM—not to be given IV |
| Methadone | 0.2-0.5 mg/kg | IV, IM |
| Morphine | 0.2-0.5 mg/kg | IV (slow administration advised to prevent histamine release), IM |
| NSAIDs DOSES SUGGESTED FOR SINGLE USE | ||
| Carprofen | 2-4 mg/kg | SC, IV |
| Ketoprofen | 2 mg/kg | SC, IM |
| Meloxicam | 0.1-0.3 mg/kg | SC |
SC, Subcutaneous; IM, intramuscular; OTM, oral transmucosal; NSAIDs, nonsteroidal antiinflammatory drugs.
* For information on repeated dosing and dosing intervals, please see details in the text.
Butorphanol (Torbutrol, Torbugesic) is one of the few analgesic drugs to have market authorization for use specifically in the cat in some countries, including the United States and United Kingdom. It is an agonist at the OP2 receptor, an antagonist at the OP3 receptor, and exhibits a ceiling effect. This is clinically relevant, insofar as increasing doses do not produce further analgesia.81 In one research model using a somatic thermal stimulus, the duration of action was approximately 90 minutes, regardless of dose,81 whereas a similar study showed large intercat variability, with antinociception lasting up to 8 hours in some cats.63 The response to butorphanol may vary depending on the source of pain. Visceral antinociception was demonstrated with 0.1 mg/kg of butorphanol, whereas somatic antinociception was unaffected in the same cats.117
A large multicenter study comparing the clinical usefulness of butorphanol with that of buprenorphine in more than 150 cats undergoing primarily, but not solely, ovariohysterectomy or castration found that buprenorphine resulted in lower pain scores for a greater duration than did butorphanol.143 Current data suggest that butorphanol is a sensible choice for acute, visceral pain (e.g., cystitis), but in light of its relatively short duration of action and ceiling effect, it is a poor choice for somatic or visceral pain that is more than transient in nature, such as would occur with invasive surgery.108
Nalbuphine (Nubain), like butorphanol, is an opioid agonist–antagonist. Little has been published regarding the use of nalbuphine in cats. One study demonstrated visceral analgesia, with intravenous doses of 0.75, 1.5, and 3 mg/kg producing similar effects that lasted between 156 and 200 minutes.117 None of these doses resulted in somatic analgesia.
Pentazocine (Talwin), another agonist–antagonist, only provided visceral analgesia when 3 mg/kg (intravenous) was administered.117 No somatic antinociception was noted with this dose, and undesirable side effects such as ataxia and apprehension were described, which suggests that this drug has little utility in cats.
Buprenorphine (Buprenex) is a partial OP3 agonist and has been widely studied in cats in both laboratory and clinical settings. It has market authorization for use in cats in some countries. Laboratory studies report varying times to onset of effect and duration of action, which appear partly related to dose and route of administration. For example, when antinociception is evaluated using a thermal threshold model, a dose of 0.01 mg/kg (intramuscular) required up to 2 hours for onset of action, and the duration of effect varied from 4 to 12 hours.110 In the same model a dose of 0.02 mg/kg (intramuscular) resulted in a quicker onset of antinociception, which was significant at 35 minutes and lasted approximately 5 hours.63 Intravenous administration of buprenorphine across a range of doses from 0.01 mg/kg to 0.04 mg/kg showed that onset of thermal antinociception time is short (15 minutes), with little difference in intensity or duration of effect. A different test of somatic analgesia (mechanical threshold) did show a dose-related effect; 0.01 mg/kg was ineffective, 0.02 mg/kg was effective but short acting, and 0.04 mg/kg had the longest duration of action.135 These authors commented that another reason for the variability among studies was significant intercat variability, a reminder that pain and the efficacy of drugs used to relieve it are unique to each individual and that individual assessment is the key to success in each patient.
Similar to the reported effects of subcutaneous hydromorphone, this route also seems less effective when a single dose of buprenorphine is used.43,134 In a laboratory setting, there was no difference in onset or duration of action (30 minutes and 6 hours, respectively) between oral transmucosal and intravenous administration of buprenorphine at 0.02 mg/kg. In a clinical trial intravenous and intramuscular administration of buprenorphine were more effective than the oral transmucosal route.43 However, this could be a result of the low oral transmucosal dose used (0.01 mg/kg). Additional information gained from these studies is the time of peak effect, which consistently occurs between 60 and 90 minutes after administration. Pain is often most intense in the immediate postoperative period. Therefore the timing of preoperative (preemptive) buprenorphine administration should be planned to meet these needs.
In several clinical studies, the analgesia produced by buprenorphine (usually given intramuscularly) in cats undergoing a variety of invasive procedures was greater in magnitude and longer lasting than that produced by several other opioids, including butorphanol, levomethadone, morphine, oxymorphone, and pethidine.33,91,129,133,143 However, it should be noted that equianalgesic doses of opioids were not necessarily used, and the methods of pain assessment were not standardized.
A sustained-release preparation of buprenorphine, available from ZooPharm (Fort Collins, Colo.), for subcutaneous administration has been evaluated in cats undergoing ovariohysterectomy. A single sustained-release dose of 120 µg/kg was as effective as 20 µg/kg buprenorphine by the oral transmucosal route given every 12 hours until 60 hours after the operation.18 The sustained-release formulation is convenient to use, and in feral cats, which are often difficult to handle after procedures, this formulation is a viable option for providing postoperative analgesia of suitable duration.
Buprenorphine is available for use in humans as a matrix patch. In cats the plasma concentrations were quite variable after application of a 35 µg/h patch, and no analgesia was evident during a 4-day period in one study.96 Until further studies are performed with different sizes of patches and perhaps using a loading dose of buprenorphine, this method of administration cannot be recommended.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree