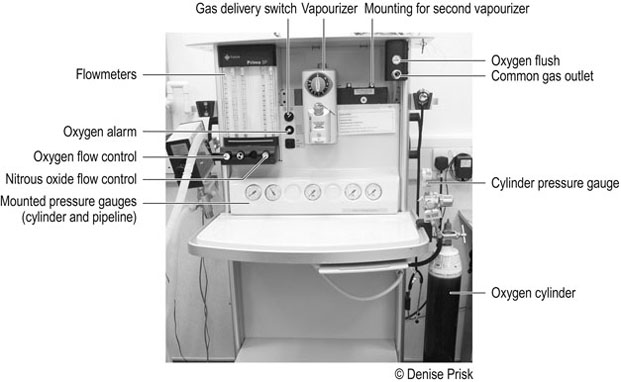
Anaesthetic machines are designed to deliver accurate amounts of carrier gases and volatile liquids in a vapour form to the patient to produce anaesthesia (Figs 6.1 and 6.2). Table 6.1 describes the parts of the anaesthetic machine. Table 6.1 Parts of the anaesthetic machine 1. Action: Turn on the spare oxygen cylinder and check that it is full. 2. Action: Turn the cylinder off and label it as full. 3. Action: Turn on the in-use cylinder or piped oxygen supply, check the contents and replace if necessary. Label the cylinder as in-use. Rationale: If the pressure reading is in the red area of the gauge, the cylinder should be changed. 4. Action: Repeat the process for the nitrous oxide cylinders or piped supply. 5. Action: Open and close the oxygen flowmeter valve. Rationale: This confirms that the ball or bobbin can move and rotate freely in its column of gas. 6. Action: Check the low oxygen alarm by turning the oxygen cylinder off and pressing the oxygen flush valve. Turn the oxygen back on. 7. Action: Check that the correct vapourizer is properly fitted and that it is full. 8. Action: Connect the correct circuit, having checked it carefully for faults. 9. Action: Connect the scavenging system. Switch active systems on. 1. Action: Check contents of gas cylinders and replace as necessary. Rationale: This ensures the machine is ready for the next use. 2. Action: Turn the oxygen cylinder off and press the oxygen flush valve until no pressure reads on the pressure gauge. Turn the piped gas supply off. Rationale: All oxygen must be flushed from the pipes. Oxidizing gases are a fire hazard. 3. Action: Wipe the anaesthetic machine with disinfectant. 1. Action: Food should be withheld for the required period of time, as necessary, depending on species, age and condition. For routine procedures in healthy adult dogs and cats, food is generally withheld for 6–12 hours. Water should be available up until the time of premedication. 2. Action: Cats should be kept inside overnight with a litter tray until ready to be taken to the practice the following morning. Rationale: This prevents the cat from disappearing and possibly missing its scheduled procedure. 3. Action: Dogs should be walked prior to admittance. Rationale: This allows the patient to urinate and defecate before admission. 4. Action: Cats must be brought to the surgery in a secure cage or basket. Dogs must wear a secure collar and be on a lead. Rationale: This minimizes the risk of escape. 5. Action: The client is given a time to arrive at the surgery. 1. Action: Check that the patient is included on the day’s list and confirm the procedure. 2. Action: Take the client and pet into a consulting room. Rationale: This is more professional than dealing with the client in a busy waiting room. 4. Action: Obtain a complete history (Table 6.2). Table 6.2 Questions to ask when obtaining a history Rationale: An accurate history is vital in order to evaluate the patient’s anaesthetic risk. 5. Action: For small mammals and exotic species, it is especially important to find out the animal’s normal routine, including its usual diet and preferred drinking method. 6. Action: Check the sex of patients admitted for neutering procedures. Rationale: It is far better to discover a mistake prior to surgery. 7. Action: Identify a cryptorchid patient prior to castration. 8. Action: Obtain a contact telephone number for the duration of the patient’s stay. 9. Action: Check the owner understands all the details, then obtain a signature on the consent form. 10. Action: Transfer the patient to a kennel, making sure it cannot escape on the way. 1. Action: Assess the function of the cardiovascular system by auscultation of the heart and palpation of the pulse. (For normal values see Table 2.1.) 2. Action: Assess the function of the respiratory system. Rationale: This is also affected by anaesthesia. Note the respiratory rate and any abnormalities. 3. Action: Palpate the abdomen. 4. Action: Palpate superficial lymph nodes. Rationale: Enlarged lymph nodes may indicate the presence of infection, allergy or neoplasia. 5. Action: Take the patient’s temperature. 6. Action: Assess pain, if relevant, prior to the administration of a premedicant. 7. Action: Take a blood sample to perform a pre-anaesthetic blood screen, if required. 8. Action: Administer a premedicant. Rationale: Premedication is performed to sedate the patient, to relieve anxiety, to enable reduced doses of anaesthetic drugs to be used and to allow a smooth recovery. In addition, analgesia is provided with the inclusion of analgesic drugs. The patient should be kept warm after premedication as many drugs used for sedation cause a drop in body temperature. Common premedicant agents are shown in Table 6.3. Table 6.3 During the induction period the patient passes from consciousness to unconsciousness. Induction is usually performed by intravenous injection, using one of the induction agents described in Table 6.4. Other methods are also possible and the advantages and disadvantages of each method are discussed in Table 6.5. Table 6.4 Table 6.5 During this period the state of unconsciousness is usually maintained with the use of an inhalant agent, delivered by means of an endotracheal tube connected to an anaesthetic breathing system and machine. In some cases an anaesthetic mask may be necessary, for example, if intubation is not possible. Total intravenous anaesthesia (TIVA) is a method of maintaining anaesthesia with the constant infusion of a suitable intravenous agent or by giving incremental doses of the agent. It is preferable that such patients undergoing TIVA are intubated to ensure the airway is maintained and protected. This also allows the administration of oxygen. The common inhalant agents are described in Table 6.6. Table 6.6 • The tongue can be held with no resistance. • There is no gagging or swallowing reflex on introduction of the tube. 1. Action: Select several endotracheal tubes of varying sizes and measure the required length against the patient’s head and neck. 2. Action: Inflate the cuff of cuffed tubes and leave for several minutes; inspect for excessive wear and check the patency of the tube. 3. Action: Lubricate the tube with water-soluble lubricant. 4. Action: The patient is restrained in sternal recumbency. An assistant extends the neck and holds the head so the nose is pointing upwards (Fig. 6.3). 5. Action: The upper jaw is held still whilst the tongue is pulled out and down so that it lies between the lower canines. Pull the lower jaw downwards by pulling the tongue down until the epiglottis can be clearly seen. Rationale: In this position, visibility of the anatomy of the pharynx is maximized (Figs 6.4 and 6.5).
Anaesthetic procedures
INTRODUCTION
THE ANAESTHETIC MACHINE
Component
Description
Function
Gas supply
In the UK, medical gas cylinders are identified by the colour of the shoulder (top part). Oxygen cylinders are black with a white shoulder, or all white. Nitrous oxide cylinders are blue. Cylinders may either be attached to the anaesthetic machine (sizes E and F) or kept remotely and gas piped to the machine (size J)
Supplies fresh oxygen and nitrous oxide to the patient
Pressure reducing valve or regulator
Placed between the cylinder/pipe and the flowmeter. Often not visible as may be incorporated into the yoke or positioned underneath the machine
Reduces the pressure of the gas leaving the cylinder to a safe working pressure
Pressure gauges
Colour-coded for different gases, these may be attached to the anaesthetic machine near the gas supply (cylinder or pipe) or incorporated into the front panel of the machine (Fig. 6.2A). As the pressure in the cylinder of non-liquid gases falls, so does the pressure reading, indicating the amount of gas remaining in the cylinder. The pressure gauge of piped gases shows the pressure in the pipelines only, not in the cylinder. A separate gauge is attached to large cylinders.
The gauge of nitrous oxide cylinders always reads full until the cylinder is empty, as it shows the pressure of the vapour above the liquid. Therefore, the pressure gauge cannot be used to assess the amount of nitrous oxide remaining in a cylinder – it must be weighed
For non-liquid gases, such as oxygen, the volume of gas remaining in the cylinder is proportional to the pressure reading on the gauge
Flowmeters (or rotameters)
Consist of a tapered glass or plastic tube, calibrated and colour-coded for different gases. A bobbin (oblong) or ball (round) should rotate freely within the tube, providing an accurate reading of the flow rate. The reading is taken from the top of a bobbin or the middle of a ball. The dot in the centre of the bobbin is to ensure it is spinning in the gas
Control and measure the flow of gas. Calibrations are in l/min. Some machines also have flowmeters which are calibrated in 100 ml/min, up to 1 litre, in addition to those in l/min (Fig. 6.2B)
Vapourizers
Modern vapourizers, such as the TEC and Penlon type, are designed to remain accurate in output despite temperature and gas flow changes. They are agent-specific
Deliver a known concentration of anaesthetic vapour to the patient
Back bar
Flowmeters and vapourizers can be attached to the back bar. This allows more than one volatile agent to be available
Supports the flowmeters and vapourizers
Common gas outlet
Location varies between anaesthetic machines
Enables connection to anaesthetic breathing systems and ventilators
Oxygen flush valve
A button or valve that allows pure oxygen to be released at high pressure and high flow, bypassing the vapourizer. Care must be taken when using this valve to deliver oxygen to a patient, to avoid barotrauma to the lungs
Provides oxygen in emergency situations and purges anaesthetic gases from the circuit before disconnection, to minimize pollution
Low-oxygen alarm
An alarm sounds when oxygen levels become low. In modern anaesthetic machines, the delivery of nitrous oxide stops when the oxygen alarm sounds, activating the nitrous oxide alarm also
Warns the anaesthetist of low oxygen levels


Procedure: Checking the anaesthetic machine before use
Procedure: Shutting down the anaesthetic machine
PATIENT PREPARATION
Procedure: Pre-anaesthetic instructions
Procedure: Admitting the patient
Question
Significance
1. How old is the animal?
The animal’s age should be on the client records but should be checked: old and very young patients may pose a higher anaesthetic risk
2. When did the animal last eat?
If food has been recently consumed, there is an increased risk of vomiting or regurgitation during anaesthesia
3. Has the animal had any previous illnesses and if so, what treatment was given?
Client records should supplement this information. Any condition involving the major body systems may increase the anaesthetic risk
4. Have there been any signs of illness in the past 24 hours? If so, what were the symptoms and has the animal recovered?
Anaesthetic risk might be increased due to dehydration, fever or electrolyte imbalance. Pathogens may also be introduced to the environment
5. How well does the animal tolerate exercise?
Poor exercise tolerance may indicate cardiovascular or respiratory problems
6. Is the patient on any medication? If so, has it been administered today?
Some drugs can alter the effects of anaesthetic agents
7. Is there any history of allergies or drug reactions?
Prolonged recovery from a previous anaesthetic or anaphylactic reactions to any medication should be noted
8. Is the patient entire or has it been neutered? If an entire female, is she in season or pregnant?
If ovariohysterectomy is to be performed on a pregnant female, surgery time may be prolonged and there might be an increased risk of haemorrhage
Procedure: Pre-anaesthetic check
Drug
Type/group
Use
Warnings
Acepromazine
Sedative/ataractic: phenothiazine
Calms the patient prior to induction and allows reduced doses of induction agent
Used with opioid analgesics to produce neuroleptanalgesia
May reduce the seizure threshold. Its use may be avoided in known epileptic patients
Boxers are very sensitive to the effects of acepromazine and it is used with caution at low dose rates in this breed
Diazepam
Sedative/ataractic: benzodiazepine
Calms the patient prior to induction and allows reduced doses of induction agent
Used as a premedicant for sick patients and those prone to seizures
Usually only produces sedation in very young or very sick patients
Medetomidine and dexmedetomidine
Sedative:
alpha-2 agonist
Used as a premedicant or in combination with other drugs to produce sedation for minor procedures. It can reduce the dose of induction agent by up to 80%. These agents also have analgesic properties. The sedative and analgesic effects are antagonized with atipamezole
Alpha-2 agonist agents cause cardiovascular and respiratory depression. They should only be used in healthy patients. Spontaneous arousal is possible and care should be exercised when using in aggressive animals
Morphine, methadone, pethidine
Analgesic:
full opioid agonist
Produce pain relief. Given prior to surgery to provide pre-emptive analgesia which leads to decreased requirements in the post-operative period
Full opioid agonist agents may cause respiratory depression, although this is rarely a problem in veterinary medicine. Morphine causes vomiting in healthy dogs
Buprenorphine, butorphanol
Analgesic:
partial opioid agonist
As above
Butorphanol provides better sedation but less analgesia than buprenorphine. Its effects are also shorter-acting
Carprofen, meloxicam
Analgesic:
non-steroidal anti-inflammatory drug
(NSAID)
May be given at premedication, after induction or during recovery, to provide multimodal analgesia
Renal toxicity and gastric irritation
GENERAL ANAESTHESIA
Induction
Agent
Effects
Warnings
Propofol
A propyl phenol agent
Rapidly metabolized in the dog by the liver, lungs and muscles. Its effects are therefore not cumulative in dogs. Anaesthesia in the dog can be maintained by giving incremental doses or by constant rate infusion – a technique known as total intravenous anaesthesia (TIVA). It is cumulative in cats, causing prolonged recovery, and should not be topped up or infused continuously
Muscle twitches are sometimes seen at induction or during recovery
A brief period of apnoea and a fall in blood pressure may be seen on induction, especially if administered quickly
Two preparations are currently available: Preservative-free, which should be discarded 6 hours after opening.
Preservative with benzyl alcohol, giving a broached shelf-life of 28 days
Alfaxalone
A neurosteroid anaesthetic agent
Non-cumulative in both dogs and cats, so can be used for TIVA without prolonging recovery time
Post induction apnoea is sometimes seen. Its occurrence can be minimized by administering the dose slowly, over 1 minute. Induction time may therefore be prolonged
Ketamine
A dissociative agent
Produces anaesthesia and profound analgesia
Combined with alpha-2 agonists, opioid analgesics and benzodiazepines to produce anaesthesia in cats, dogs, rabbits, small rodents, birds and reptiles
Cannot be used alone as it causes muscle rigidity and central nervous system stimulation
Hallucinations and emergence phenomena are often seen during recovery in the cat
Method
Advantages
Disadvantages
Mask induction
A tightly fitting clear plastic mask with a diaphragm is placed over the animal’s face. 100% oxygen is administered for several minutes; this maximizes tissue perfusion and allows the patient to adjust to the mask. The anaesthetic concentration is then gradually introduced
Used to administer oxygen and inhalant agents when endotracheal intubation is not possible
Useful for small mammals and birds
May be very distressing for the patient
The airway is not protected and obstruction can occur
Masks increase mechanical dead space
Atmospheric pollution is a significant hazard
Chamber induction
The conscious patient is placed inside an anaesthetic induction chamber, which should be large enough for the animal to lie with its neck extended. Oxygen and the inhalant agent are delivered via a gas inlet. Waste gases are scavenged via an appropriate outlet. The patient is removed when it loses the ability to stand
Induction chambers are useful for small mammals and uncooperative patients
Only suitable for small patients
Risk of vomiting
Cardiopulmonary function cannot be monitored
Atmospheric pollution occurs when the chamber is opened
Intravenous
The induction agent is injected into a peripheral vein, over the recommended period of time, depending on the agent being used
Smooth and usually rapid induction
Most induction agents can be given to effect so minimal quantities are used
Patients must be restrained well
Perivascular injection can be avoided by placing an intravenous catheter before induction
Intramuscular
This method can only be used for certain drugs such as those used in combination mixtures
Technically easier than intravenous injection
Useful in fractious patients when intravenous access is not possible
Dosage is according to weight; it is therefore not easy or possible to dose to effect
Slower onset of anaesthesia
Maintenance
Agent
Properties
Effects
Warnings
Isoflurane
Low solubility so quick onset of action
Rapid induction and recovery
Good muscle relaxation
Little effect on cardiac output
Respiratory depression
Hypotension
Approximately 0.2% is metabolized by the liver; the rest is exhaled unchanged
Sevoflurane
Very insoluble so less potent than isoflurane with very quick onset of action
Rapid and smooth induction and recovery
Less odour than other inhalant agents so useful for mask and chamber inductions
Dose-dependent decrease in cardiac output
Respiratory depression and hypotension as for isoflurane
Decomposes in the presence of soda lime – higher than normal fresh gas flows may be needed (unless using soda lime formulated for low-flow usage)
Desflurane
The least soluble of all inhalant agents
Extremely rapid onset of action and recovery
Similar cardiovascular depression as other agents
Pungent and irritant to airways so not suitable for induction by inhalation
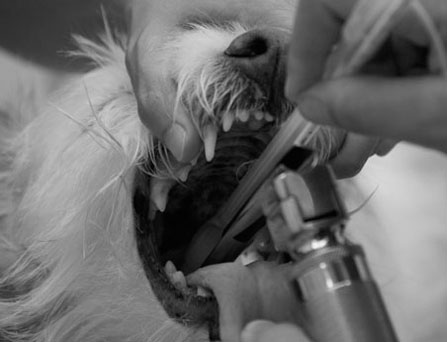
ENDOTRACHEAL TUBES
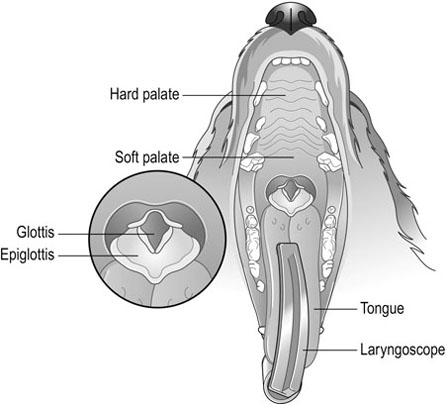
Procedure: Intubation of a patient
Equipment required
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


