Chapter 17
Anaesthesia of zoological species (exotic pets, zoo, aquatic, and wild animals)
Stephen J. Divers
Preanaesthetic sedation and analgesia
Regional anaesthesia and local blocks
Recovery and postoperative care
Lagomorphs – rabbits (Oryctolagus cuniculus) and hares (Lepus europaeus)
Rodents – rats (Rattus norvegicus), mice (Mus musculus), guinea pigs (Cavia porcellus), hamsters and gerbils (Mesocricetus auratus and Gerbillidae)
Mustelids – ferrets (Mustela putorius furo), mink (Mustela lutreaola), European otter (Lutra lutra), stoat or short-tailed weasel (Mustela ermine), polecat (Mustela putorius), badger (Meles meles)
Insectivores – European hedgehogs (Erinaceus europaeus), African pygmy hedgehogs (Atelerix albiventris)
Preanaesthetic stabilization, sedation and analgesia
Regional anaesthesia and local block
Preanaesthetic stabilization, sedation, and analgesia
Regional anaesthesia and local block
Preanaesthetic stabilization, sedation, and analgesia
Introduction
The problems involved in anaesthetizing such diverse taxa as birds, reptiles, fish, and exotic mammals present unique and varied challenges. Dangerous animals (e.g. venomous snakes, large carnivores, etc.) are often impossible to evaluate and examine thoroughly prior to induction for reasons of human safety. Laboratory animals (e.g. rabbits and rodents) are often healthy and, when anaesthetized for experimental purposes, it is particularly important that methodologies have little or no influence on the result of the study. Conversely, pet rabbits and rodents are often unhealthy and considerably older. The techniques to be described in this chapter are those which the author has found to be satisfactory in general practice for most clinical purposes in the various species of animal presented. In some cases, specialized drugs and equipment not commonly found in general practice are required, which only adds to the importance of advanced planning. Readers are directed to specialized texts for more in-depth descriptions as only brief overviews can be provided here (Brown, 1993; Heard, 2004; Edling, 2006; Schumacher & Yelen, 2006; West et al., 2007).
Small mammals
With over 10 million pet rabbits (Oryctolagus cuniculus), ferrets (Mustela putorius furo), and rodents (order Rodentia) in the USA, these small mammals represent the third largest group of companion mammals, after dogs and cats (AVMA, 2007). Although similar demographic data have not been located for Europe, this group nevertheless represents an expanding component of small animal practice, with many clients expecting the same level of medicine for them as for more traditional pet species. Many diagnostic and surgical procedures warrant anaesthesia, therefore, acquiring skills for competent anaesthesia is an indispensable skill for the exotic animal practitioner. Short anaesthetic procedures are commonplace for completing tasks such as thorough examination, phlebotomy, radiography, or other short diagnostic/therapeutic procedures. In addition, many small mammal clinical problems necessitate surgery. Readers are directed to detailed reviews that are available on the subject, as personal preferences will largely be presented here (Heard, 2004, 2007a,b; Carpenter, 2005). Practitioners are also finding that sedation, especially when combined with local anaesthesia, is often a viable option to general anaesthesia for such procedures as sample collection and catheterization.
Although rodents and other small mammals are anaesthetized in large numbers for laboratory procedures with apparently few serious problems, when similar species are anaesthetized for clinical purposes, the mortality is higher. The cause of the higher clinical mortality probably results from unfamiliarity with the species and the generally poor health of many companion animals. Therefore, success can be improved by:
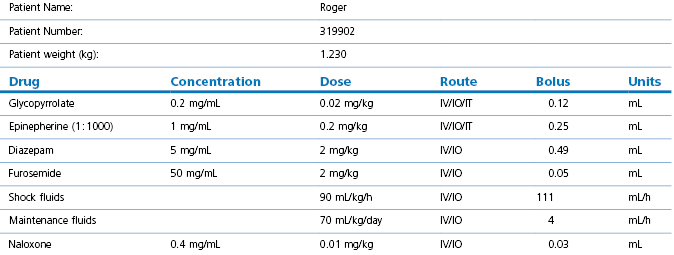
2.Calculate emergency drugs, or consider a dedicated emergency drug chart (Table 17.1). For critical cases, prepare individual emergency drug doses before induction
There are some basic principles that apply when using general anaesthesia in small mammalian patients in addition to the sound, basic principles of domestic animal anaesthesiology. These additional comments are based on the fact that exotic mammals are often small in size, have high metabolic rates, have high body surface area : volume ratios and are prone to hypothermia, are often catecholamine-driven prey animals that ‘stress’ easily, are typically presented in advanced stages of disease (often respiratory) with little respiratory or cardiovascular reserve, and have anatomy that challenges endotracheal intubation and intravenous access. However, there are solutions that can help address these problems (Table 17.2). It should be kept in mind that patients under sedation alone must still be carefully monitored.
Table 17.2
Common problems, complications, and possible solutions associated with small mammal anaesthesia
| Problem | Potential complication | Possible solution |
| Small size | Mechanical obstruction of airway due to positioning; compression of thoracic cavity during handling/surgery | Be careful to keep head/neck extended; do not place heavy drapes, equipment or rest hands/arms on the animal during anaesthesia and surgery |
| High metabolic rate | Hypoglycaemia, especially if prolonged fasting prior to procedure; rapid drug metabolism; higher fluid requirements; unexpected drug effects of interactions (e.g. atropine and tiletamine in rabbits) | Plan to be able to deliver higher fluid requirements and administer fluids pre-, intra- and postoperatively; consider dextrose in IV fluids if hypoglycaemic; minimize fasting time; utilize drug dosages based on species-specific pharmacokinetic studies; know the species idiosyncrasies, avoid drug contraindications, be prepared to reverse drugs if undesirable effects are appreciated, be prepared to provide respiratory support |
| High surface area : volume ratio | Hypothermia (small animals lose heat more readily) especially during abdominal/thoracic surgery; hypothermia can lead to decreased anaesthetic requirements, prolonged recovery, bradycardia, and terminal ventricular fibrillation | Provide warmth from the point of induction; monitor body temperature carefully and frequently; plan for different types of supplemental heat during anaesthetic episode and use them simultaneously (radiant source, heating pad, warm fluids, warm air); administer heat prior to animal becoming hypothermic and continue until the animal can thermoregulate |
| Catecholamine-driven prey animals | Stressed prey species release endogenous catecholamines that sensitize the myocardium (among other effects); this effect is much more pronounced in certain species (especially rabbits) | Minimize handling of untamed animals preoperatively; plan for hospital environment to be stress-free (shelter boxes, quiet wards away from predators); premedicate patient with anxiolytics prior to induction; utilize strategies such as rapid induction agents and induction chambers (instead of facemask) to minimize handling |
| Present with underlying cardiovascular disease | Small thorax, limited tidal volume, little respiratory or cardiovascular reserve | Perform a preanaesthetic evaluation; minimize anaesthetic time through efficiency (not rushing); carefully monitor patient (ETCO2, SpO2, blood pressure, blood gases); be able to ventilate animal; have crystalloids, colloids, dopamine/dobutamine, and emergency drugs available |
| Difficult to intubate | No control over ventilation; potential for hypoxaemia and hypercapnia | Study anatomy of the animal prior to scheduling procedure and research intubation methods; practice on cadavers; many small mammals can be safely intubated with practice and proper equipment; if unable to intubate, realize limitations of lack of control of airway, keep procedure short; utilize well-fitting mask and appropriate lab animal anaesthetic circuit (not a traditional non-rebreather or T-piece circuit) |
| Difficult to gain vascular access | Unable to administer maintenance fluids during anaesthetic procedure to maintain proper blood pressure and blood flow to vital organs; unable to administer emergency agents if needed | Study anatomy of the animal prior to scheduling procedure and research catheterization methods; many small mammals can be catheterized, either IV or IO with practice and proper equipment; if unable, administer maintenance fluid requirements SC before the procedure (or at induction), and repeat just prior to recovery |
Many small mammals become distressed by handling, increasing the risk of physical damage and of adrenaline release leading to problems under subsequent anaesthesia. Respiratory rate should be recorded before the animal is disturbed and removed from the cage. The risk of physical damage is considerably reduced by proper handling. Many rabbits and rodents can be secured using the dorsal scruff, while a towel over the eyes can further reduce stress and struggling. Small mammals can be placed into a small bag or box and accurately weighed. Adequate preanaesthetic examination is often difficult but many have respiratory disease so oxygen should be available even if only injectable agents are to be used. The high metabolic rate of these small mammals means that they require an almost constant supply of food, so preanaesthetic fasting should not exceed 2 hours. In general, the cardiac sphincter of most rabbits and rodents prevents regurgitation, while the majority of the gastrointestinal ingesta are located in the hind-gut such that prolonged fasting would be required to reduce volume, and likely lead to hepatic lipidosis. The main purpose of short-term fasting is, therefore, to reduce food material within the oral cavity. There is no need to curtail the water supply up to the time of induction of anaesthesia; however, fasting typically requires not only the removal of all food but also all bedding materials that may be chewed. During anaesthesia, small mammals are particularly prone to hypothermia (<37.8°C, <100°F) and precautions to avoid this should be taken from the time of induction. Removal of hair and wetting (particularly with alcohol-based preparations) should be kept to a minimum; the use of overhead radiant heat sources, warmed-water and warmed-air blankets should be employed at induction, as well as during maintenance and recovery periods to maintain core temperatures (Fig. 17.1). Heat loss can be considerably reduced by wrapping the animal in foil or bubble wrap during non-surgical procedures, while the application of clear plastic surgical drapes helps maintain temperature during surgery. When inhalation agents are used, carrier gases also contribute to cooling effects, thus gas flows should be adequate but not excessive. In-line gas warmers can be used as long as increased circuit resistance is overcome. Intravenous or intraosseous catheterization should also be considered routinely and, in cases where this is likely to prove difficult, subcutaneous fluid therapy should be instituted prior to induction and repeated during anaesthesia (Fig. 17.2). Maintenance of hydration status and circulation, ventilation and temperature appear to be key. Adequate monitoring of the animal’s condition, including cardiac and respiratory function is essential until recovery is complete.
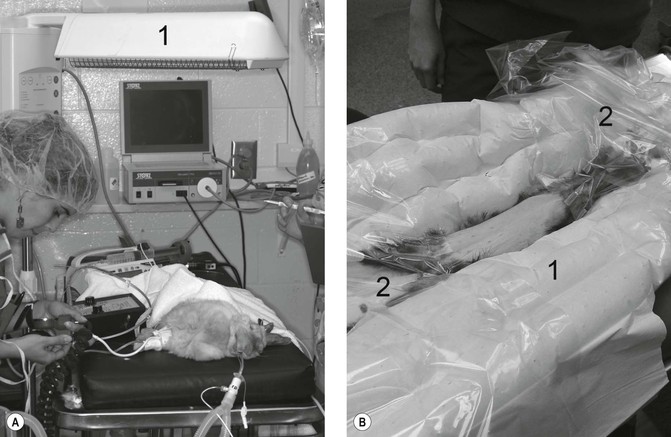
Figure 17.1 Warming devices. (A) Radiant heating element (1) positioned above an anaesthetized rabbit; (B) ferret positioned on top of a warm forced air blanket (1), with additional clear drapes (2) to maintain temperature following aseptic preparation of the abdomen. Note blood pressure measurement on the rabbit is Doppler ultrasound technique. Courtesy of Dr Stephen Divers, University of Georgia.
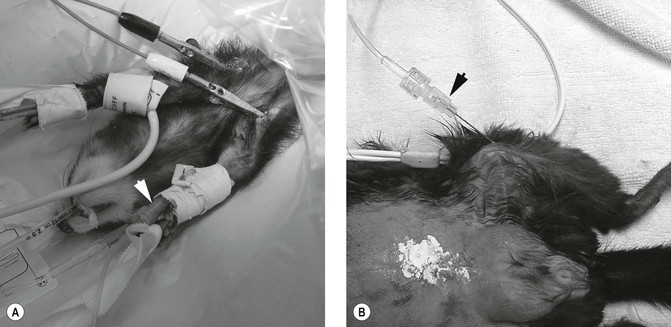
Figure 17.2 Fluid therapy. (A) IV fluid therapy via a cephalic catheter (arrow) in a ferret; (B) IO fluid therapy via a spinal needle placed into the tibia (arrow) in a guinea pig. Courtesy of Dr Stephen Divers, University of Georgia.
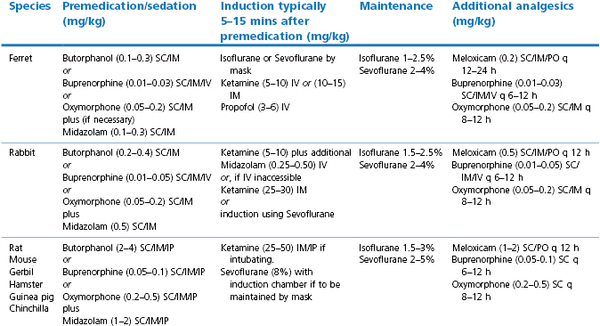
The use of anticholinergic premedication is controversial as in other species, but as small airways can be easily blocked by saliva or mucus, their use may be desirable. Glycopyrrolate is preferred for rabbits as they possess increased atropinase activity that reduces the efficacy and duration of atropine. The author prefers an opioid and benzodiazepine combination for premedication, and these drugs can be given by intramuscular or, in smaller animals, by subcutaneous injection (Table 17.3). General anaesthesia is preferred for most purposes and may be induced and maintained with volatile agents, induced with injectable drugs and maintained with volatile agents, or maintained with injectable drugs alone. There are many different protocols available and only those routinely used and preferred by the author are presented here (Table 17.3). A more complete listing can be found in the references (Brown, 1993; Carpenter, 2005; AVMA, 2007; Chinnadurai et al., 2010).
Preanaesthetic sedation and analgesia
Midazolam is commonly used in human and traditional pet medicine for purposes of preanaesthesia and sedation. It has a wide margin of safety in many species. When combined with an opioid, effects are synergistic, allowing a reduction of the amount of either drug (see Table 17.3). Effects in exotic species are promising, and this author has not observed severe adverse effects in any species in which it has been utilized (avian, exotic mammals including rabbits, ferrets, rodents, primates, carnivores, and others). Effects are variable, from slight decrease in activity to lateral recumbency. Effects appear to be more pronounced in the rabbit and ferret and in compromised patients, and less pronounced in rats and mice unless higher dose rates are used. These effects are likely related to species variability in response and the varying dose rates suggested for different species/groups. However, with the availability of inexpensive flumazenil, the sedative effects can, if deemed necessary, be reversed postoperatively. In all cases, patients still react somewhat to handling and noxious stimuli. When midazolam is combined with an opioid, greater sedation and analgesia result. Depending on overall patient condition and goal of the sedation procedure, additional sedation can be provided with subanaesthetic dosages of ketamine (see Table 17.3). If additional immobilization is essential, low concentrations of inhalant gas can be considered. This combination of drugs for sedation allows overall reduction of required inhalant gases, decreasing risk associated with the use of general anaesthesia.
Regional anaesthesia and local blocks
It should be kept in mind that use of sedation and manual restraint alone is inappropriate in clinical practice for any procedure expected to produce discomfort. An exception may be in a calm patient with judicious and efficient use of local anaesthesia in the form of a local or regional block. In addition, when used in combination with general anaesthesia, the use of local blocks can help reduce general anaesthetic requirements. For example, epidural blocks for pelvic orthopaedic surgery, and dental blocks for tooth extractions appear to be particularly worthwhile. Local anaesthetic drugs should be carefully calculated and diluted if necessary to avoid overdose. The most commonly utilized agents for local block are 1 mg/kg lidocaine and 1 mg/kg bupivacaine. The onset of action of lidocaine is rapid but short lived, while bupivacaine is slower to act but, in some species, may provide analgesia for up to 6–8 hours. Lidocaine is painful when injected and should therefore be buffered with bicarbonate unless the animal is unconscious.
Inhalation anaesthetic agents
The most popular agents for use in these species are isoflurane and sevoflurane. Halothane is still available in some countries and can certainly be used, albeit with a reduced margin of safety. Mask induction can lead to handling stress, particularly when using agents other than sevoflurane, and the use of an induction chamber, although preferable, in some individuals does lead to greater volatile drug use, and often greater environmental contamination unless active scavenging is available. Several such chambers are commercially available but they are relatively easy to improvise and there is no reason why they should not be available in most general veterinary practices. Induction of anaesthesia with inhalants provides very little time for intubation, unless the operator is highly skilled, and therefore, once induced, anaesthesia is generally maintained by administering the volatile agent in oxygen through a T-piece or similar low-resistance breathing system and a facemask. Rodents and other mammals <1 kg benefit from a dedicated rodent circuit (Fig. 17.3). Unlike the T-piece or similar circuits used for cats and dogs that rely on active inspiration for taking in gas, a dedicated rodent circuit forces gas into the oral cavity and this positive pressure improves ventilation. These circuits including suitable facemasks are commercially available, or can be made from plastic syringe barrels and rubber gloves. Facemasks should incorporate a close-fitting rubber skirt to reduce leaks that cause atmospheric pollution. Some form of passive or active scavenging of gases should be used.
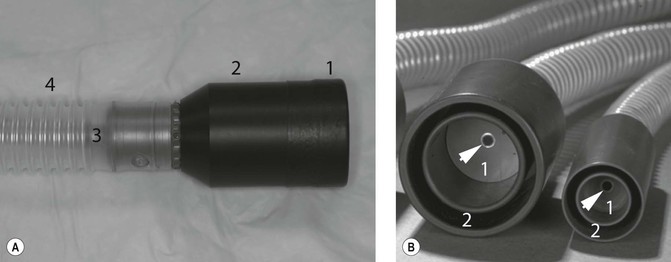
Figure 17.3 Dedicated rodent masks and circuits. (A) The rubber skirt (1) ensures that the mask (2) is secure around the rodent’s head, a small diameter tube (3) delivers gas as a jet into the oral cavity while the large diameter outer tube (4) provides a low resistance path for expired gases. (B) Close-up view of rodent masks demonstrating the small diameter gas delivery (arrow) to the breathing chamber (1), and the outer tube (2) that carries away expired gases. Courtesy of Dr Stephen Divers, University of Georgia.
Injectable anaesthetic agents
Theoretically, any injectable anaesthetic can be used in small mammals and usually the necessary doses for healthy animals are well known from the original developmental work in laboratory animals carried out by the company that marketed the drug. However, practical limitations are set by the possible methods of administration. In some animals with easily accessible veins (e.g. in rabbits), drugs such as propofol or rapidly-acting barbiturates can be used as in cats and dogs (although the duration of effect is generally much shorter). Where IV injection is more difficult, drugs can be given by IM, IP, or SC routes. The most popular combinations of drugs are the neuroleptanalgesics or mixtures incorporating ketamine. There are marked differences between species responses and, even within one species of animal, many drug actions may be unreliable, such that a given drug producing deep anaesthesia in one animal may only provide some sedation in another of the same species.
Ketamine
Ketamine has the advantage that it is effective no matter what the route of administration. Doses required and efficacy vary greatly between the various species. Lower doses may be used for sedation and immobilization for non-surgical procedures. As in other species of animal, ketamine is used in combination with drugs such as the benzodiazepines (diazepam or midazolam) and/or α2-adrenoceptor agonists (xylazine or medetomidine) in order to reduce the dose of ketamine, improve muscle relaxation and to increase the effectiveness of the dissociative agent as an anaesthetic. Many of the animals seen in clinical practice are ill and/or aged, and therefore potent α2-adrenoceptor agonists like medetomidine and dexmedetomidine are usually avoided. The author prefers to induce anaesthesia using intravenous ketamine, often in combination with additional benzodiazepine, as this induction provides more time for endotracheal intubation, compared to inhalant induction.
Neuroleptanalgesia
Although most commercially available neuroleptanalgesic combinations can be used, the mixture of fentanyl and fluanisone (Hypnorm®) has proved to be the most popular in the UK, and it can be administered by any route. The dose of fentanyl in Hypnorm® is high, resulting in a prolonged length of action and, occasionally, in respiratory arrest. Combinations of Hypnorm® with diazepam or midazolam give better muscle relaxation and allow a reduction of some 50% in the dose of Hypnorm®. If anaesthesia becomes too deep, the fentanyl component may be antagonized with naloxone (0.01 mg/kg). Buprenorphine can be used to antagonize the fentanyl in the drug combination. The technique is termed sequential analgesia and relates to the selective displacement of a pure agonist with a partial agonist/antagonist in the hope of reducing respiratory depression and sedation while maintaining analgesia.
Other agents
A mixture that is often used, although unlicensed, is known as the ‘Hellabrunn Mixture’. It was developed primarily for administration to zoo animals and is prepared by adding 4 mL of ketamine (100 mg/mL) to a vial of dry xylazine (500 mg). This yields a stable injectable solution containing xylazine 125 mg/mL together with ketamine 100 mg/mL. Its stability means that it is immediately available and it is relatively safe for the administrator. Ketamine and medetomidine/dexmedetomidine combinations are also very effective, but their use is best restricted to young healthy animals.
Propofol has been recommended for intravenous use in rabbits, rats, mice and hamsters, however, its short duration of action combined with apnoea necessitates rapid intubation. Alfaxalone has proven useful in some species when given IV. Pentobarbital and thiopental may be used but given their prolonged sedation and respiratory depression, they cannot be recommended for clinical use. The shorter acting methohexital appears safer and has been used in various small mammals (Smith, 1993).
Ventilation
This author prefers to connect all intubated small mammals <8 kg to a pressure-cycled ventilator (e.g. Small Animal Ventilator VT-5000, BASi Vetronics, USA, or SAV03 Vetronics, UK). The ventilator is routinely set to the non-rebreathing passive circuit, with active ventilation selected if required (Fig. 17.4). This has the advantage of being able to select the mode of ventilation by flicking a switch and without changing circuits. Ventilation is used whenever end-tidal CO2 (ETCO2) rises above 45 mmHg or SpO2 falls below 95%. Ventilation is used routinely for laparoscopy involving abdominal insufflation with CO2. This inexpensive pressure-cycled ventilator is simple to use and has a proven track record in exotic mammal practice. Depth and frequency of ventilation are initially set to mimic preanaesthetic respiration, but are modified to maintain ETCO2 readings of between 35 and 45 mmHg.
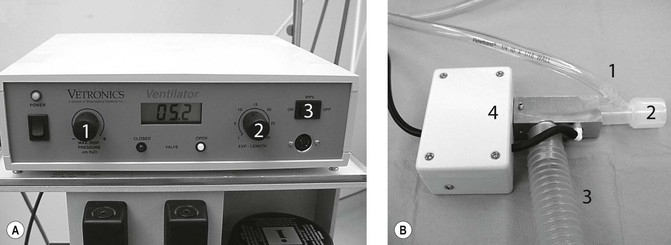
Figure 17.4 Pressure-cycled ventilator. (A) Main control unit featuring maximum inspired pressure control (1), expiration length (2), and passive/ventilator switch (3); (B) patient delivery system with gas inlet (1), endotracheal tube connector (2), waste gas removal (3) and solenoid switch (4). Courtesy of Dr Stephen Divers, University of Georgia.
Monitoring
The primary goal of anaesthetic monitoring should be to minimize morbidity by preventing, identifying, and correcting hypotension, bradycardia, arrhythmias, hypoxaemia, hypercapnia, and metabolic disturbances. Although the size and anatomy of some small mammal patients often preclude some anaesthetic monitoring techniques, this should not discourage the practitioner from including the following steps:
2.Record the precise time of premedication/induction. This step is often overlooked but it is important to know how long an animal has been anaesthetized, and to maximize efforts in expediting procedures.
3.Gauge the depth of anaesthesia by observation of mentation and reflexes.
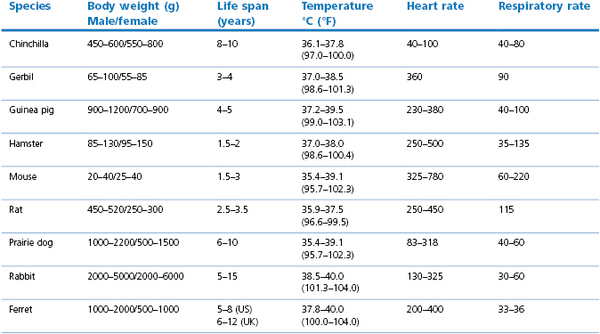
4.Utilize simple monitoring techniques rather than elaborate systems that are prone to failure. First, take a heart rate and note cardiac rhythm with a simple stethoscope and note the depth and frequency of ventilation, and judging the information based upon preanaesthetic values (Table 17.4).
5.Utilize equipment that will give you the most information with least problems, for example, ultrasound Doppler probe for heart rate and rhythm, end-tidal capnography, and ECG are easy to use. Pulse oximetry can be temperamental but still useful when a reliable pulse wave is obtained (irregular or poor pulse wave leads to untrustworthy readings that must not be relied upon). Pulse oximetry readings (SpO2) <90% is taken to correlate with hypoxaemia PaO2 ≤60 mmHg. Indirect blood pressure readings taken from the radial artery of the forelimb using a sphygmomanometer and ultrasound Doppler have been shown to be accurate and reliable compared to direct arterial blood pressures in rabbits (Ypsilantis et al., 2005). A 22 or 24 gauge catheter inserted in an ear artery is used for invasive blood pressure monitoring in rabbits.
Recovery and postoperative care
Recovery and the immediate postoperative period can be just as critical as the maintenance period in ensuring that your small mammal patient recovers completely. Indeed, the point of initial recovery and extubation can often be the most critical stage of the anaesthetic procedure, because critical support mechanisms have been withdrawn. In general, the following steps should be taken for patient support:
2.Maintain oxygen administration after extubation until fully conscious.
4.Monitor the animal frequently and administer additional fluids or antagonists as and when needed.
Postoperative analgesia is important and must not be neglected. It should be a continuation/modification of the pre-emptive analgesic protocol employed for premedication. Some opioid drugs are suitable and other methods utilizing local analgesics should be considered. It is regrettable that the rat, which has probably contributed more than most animals to advances in medical and veterinary sciences, still is ignored in many laboratories in circumstances where postoperative analgesia would be regarded as essential for other animals. Meloxicam is a preferential COX-2 inhibitor that has been tried and tested in laboratory rodents and, although multidose pharmacokinetics have yet to be determined, the author uses this analgesic routinely with good effects.
Monitor temperature regularly and remove from incubators once normothermic. Some mustelids, especially otters, appear prone to anaesthesia-induced hyperthermia that can be fatal if uncontrolled.
Lagomorphs – rabbits (Oryctolagus cuniculus) and hares (Lepus europaeus)
Rabbits and hares need to be handled carefully as they tend to panic if placed on slippery surfaces. These animals are best held for injection wrapped in a towel in the arms of an assistant or placed in a restraining box. A rabbit or hare struggling against forcible restraint may fracture a vertebra, so any restraint technique used should only entail the minimum of force. The animal should be caught by grasping the scruff of the neck firmly and pressing down on a flat surface until it relaxes, then it may be lifted by supplementing the neck grip with support for the hindquarters. Rabbits, especially when kept as pets, can be calmed by scratching behind the ears and stroking the back. Respiratory problems, usually due to various bacteria including but not limited to Pasteurella, are common in rabbits that may appear to be healthy. Auscultation of the lungs for diagnosis is not easy and many authorities advise conscious thoracic radiography prior to general anaesthesia so that owners may be warned of the anaesthetic risks associated with the presence of lung disease.
Intramuscular injections are made into the quadriceps or triceps, epaxial or lumbar muscles. Intravenous injections can be most easily given into the marginal ear, cephalic, and lateral saphenous veins. Intravenous injection is greatly facilitated by the use of a restraining box that leaves the ears accessible, or good quality restraint following sedation. Rabbits produce atropinase, which rapidly inactivates atropine, so to be effective doses of this agent must be high (1–2 mg/kg) and repeated every 15–20 minutes. Alternatively, glycopyrrolate (0.01–0.02 mg/kg) is preferred as an anticholinergic.
Although IV anaesthetic agents can be used to induce anaesthesia in rabbits they are not good for maintaining anaesthesia for even very small incremental doses may cause death through respiratory arrest. Induction of anaesthesia is satisfactory with methohexital (5–10 mg/kg), alfaxalone (2–8 mg/kg), propofol (3–6 mg/kg), or ketamine (10–15 mg/kg) and midazolam (0.5–1.0 mg/kg) given IV to effect. These agents can be given IV through a 21 gauge or 23 gauge catheter placed into an ear, cephalic, or lateral saphenous vein. The ketamine/midazolam combination is preferred by the author as it provides a longer duration of action for placing an endotracheal tube. Diazepam injected into an ear vein may cause tissue necrosis.
Induction of anaesthesia is usually quiet with sevoflurane administered in O2 1–2 L/min through a facemask from a non-rebreathing system. Induction with isoflurane is facilitated when the animal is first heavily sedated and the concentration of isoflurane increased in small step increments up to 3 or 4%. Alternatively, the rabbit can be placed in an induction chamber or plastic box with a charcoal canister on the outlet for scavenging and isoflurane in O2 introduced into the box until the animal is unconscious. Inhalation agents are eliminated rapidly after the mask is removed and leave little time for the process of endotracheal intubation.
Endotracheal intubation is relatively difficult because of the long, narrow oropharynx and long incisor teeth limiting access through the mouth. The tongue is thick, fleshy, friable, and easily torn. The soft palate is long and the epiglottis is large. In all cases, it is essential to have an adequately anaesthetized patient that is completely relaxed – attempts to intubate a semiconscious small mammal are likely to cause damage and fail. Endotracheal intubation is accomplished by either (1) direct view of the larynx using a straight, premature human infant blade (Wisconsin 00) or endoscope, or (2) blindly. A semi-rigid stylet can be used as a guide to aid in the passage of the endotracheal tube. Tubes of 2.0–4.0 mm internal diameter are suitable for use in most rabbits. Direct view of the larynx is considered the gold standard in human and veterinary medicine, and typically enables a larger endotracheal tube to be placed compared to blind techniques (Fig. 17.5). Placement of the largest possible tube becomes especially critical in smaller animals where significant decreases in airway resistance and gas flow can be obtained (see Table 17.4). The chances of successful intubation in small rabbits and rodents can be maximized by:
1.Ensuring adequate anaesthesia (see Table 17.3). Ketamine and midazolam anaesthesia will provide 5+ minutes of good restraint and relaxation for intubation (compared to 20–40 seconds following inhalant induction).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree