Chapter 15
Anaesthesia of the dog
Evaluation of the significance of disease
Sedative and analgesic premedication
Termination of neuromuscular block
Ventricular dysrhythmias, myocardial ischaemia, myocardial contusions
Bronchoscopy, transtracheal wash (TTW), bronchoalveolar lavage (BAL)
Dorsal hemilaminectomy and ventral cervical decompression
Myelography, computed tomography
Hit-by-car (HBC, RTA), high-rise syndrome (fell from a great height)
Epidural and intrathecal block
Infiltration; Soaker catheters
Intravenous regional analgesia (IVRA)
Mandibular and maxillary nerve blocks
Paravertebral nerve block (brachial plexus)
Peroneal and tibial nerve blocks
Introduction
Within the last 10 years, there has been an explosion of new information about different anaesthetic agent combinations that can be used for sedation and anaesthesia in dogs. Anaesthetic protocols can still be straightforward for short anaesthesia for elective procedures but there are more choices for anaesthetic management of increasingly complex and more protracted medical and surgical procedures. Monitoring equipment has become more available for veterinary practice, enabling more precise control of the animal’s physiological status and reducing intra- and postanaesthetic complications. There has been a resurgence in the use of nerve blocks in combination with general anaesthesia in concert with increased use of opioids not only to provide analgesia during surgery but with the intention of controlling pain after anaesthesia. This management goal is based on the desire to improve animal welfare but may have added benefits in more rapid recovery, shorter hospitalization time, and improved client satisfaction.
Sedation and analgesia
General principles
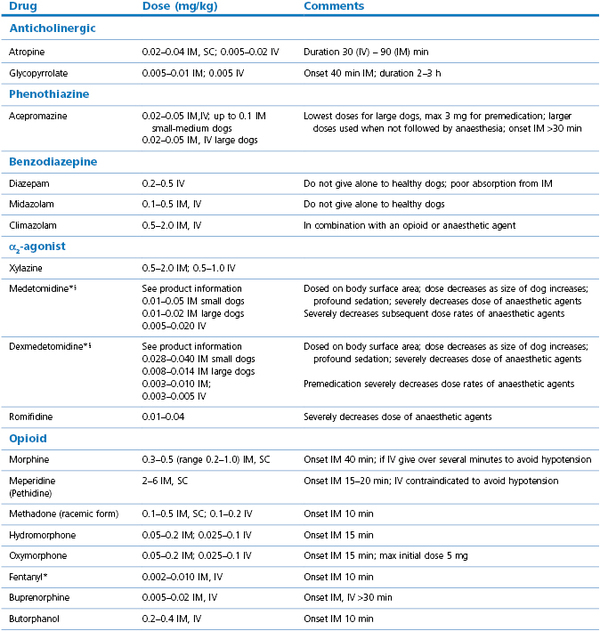
Selection of a drug or drugs for sedation depends on the purpose for which it is intended and pharmacological details of opioids and sedatives are given in Chapters 4 and 5. Mild behaviour modification can be achieved with a phenothiazine such as acepromazine whereas moderate to heavy sedation is better obtained using an α2-agonist, such as medetomidine or dexmedetomidine with an opioid, or a neuroleptanalgesic mixture such as acepromazine with morphine or methadone. Intravenous administration produces a more rapid onset and intense effect of a shorter duration than IM administration and, generally, drugs injected IV are at lower dose rates. Dose rates chosen may depend on whether the dog is to be only sedated or whether sedation is to be followed by general anaesthesia. When the drugs are used for premedication to anaesthesia, dose rates are often considerably lower so as to minimize cardiovascular and respiratory depression. Some individuals are sensitive and some resistant to anaesthetic agents and unusual responses should always be recorded in the medical record for valuable information at the dog’s next visit.
Many minor procedures can be performed when dogs are sedated and the addition of local anaesthesia allows surgical procedures, depending on the type of nerve block used. Some procedures can be satisfactorily accomplished under only sedation while others are better done with the dog under general anaesthesia, for example dental prophylaxis where general anaesthesia including endotracheal intubation is recommended (American Veterinary Dental Association, 2004). Sedation only for this procedure limits effective cleaning and the absence of an endotracheal tube increases risk of aspiration of water and debris.
Phenothiazines
Acepromazine is a commonly used phenothiazine for sedation in dogs. Use of a 2 mg/mL solution facilitates more accurate dose administration than when a stronger solution is used. When not commercially available, a 2 mg/mL solution can be made by adding 60 mg of acepromazine to a 30 mL bottle of sterile saline, after first removing an equivalent volume of saline. The bottle should be wrapped to protect the solution from the light. The response to acepromazine is not uniform and depends on the animal’s temperament, physical condition, and breed. Some giant breeds, for example, the St Bernard, Newfoundland, and Swiss Mountain dog appear sensitive to the drug and may become recumbent and reluctant to move following doses of 0.03 mg/kg. Some dogs of the Boxer breed will ‘faint’ when given acepromazine and it should be given in only small doses, ± an anticholinergic, or avoided completely in this breed.
The cardiovascular actions of acepromazine are minimal in healthy dogs but are potentiated by hypovolaemia, azotaemia, and old age. The dose rates for acepromazine decrease with increasing size of the animal; for IM administration in small dogs, 0.05–0.1 mg/kg is usually sufficient, for dogs 10–20 kg, 0.05–0.07 mg/kg, for dogs >20 kg, 0.02–0.05 mg/kg, with a maximum dose of 3 mg for most large dogs (Table 15.1). Occasionally, dogs are given 5 mg acepromazine with an opioid to induce profound sedation. Note that these doses are lower than those recommended on the product data sheets but increasing dosage rarely increases the sedative effect. The action of acepromazine is mild mood alteration and may be negligible in aggressive or excited dogs. In general, concurrent administration of an opioid with acepromazine results in significantly greater sedation.
Thirty minutes is usually required after IM injection for appreciable effects to be seen, including calming and protrusion of the third eyelid over the cornea. One investigation found no difference in sedation scores in dogs administered acepromazine, 0.025 mg/kg, and morphine, 0.3 mg/kg, IM at four different sites: the cervical epaxial muscles at the level of C3 and 2–3 cm off midline; the triceps brachii halfway between the elbow and scapula; the middle gluteal muscle injected midway between the greater trochanter of the femur and wing of the ilium; and the quadriceps femoris halfway between the femorotibial joint and the greater trochanter of the femur (Self et al., 2009). The development of sedation at 30 minutes after injection was independent of injection site and the degree of sedation was less at 20 than 30 minutes. Another common site for IM injections in dogs are the epaxial lumbar muscles. Drugs may have incomplete absorption when injected SC but a comparison of IM and SC administration of acepromazine, 0.03 mg/kg, and buprenorphine, 0.02 mg/kg, in clinical patients revealed no difference in sedation assessed after 60 minutes, and no difference in the dose of propofol required for endotracheal intubation (Gurney et al., 2009). Although some effect may be noticed by 5 minutes after IV injection of acepromazine, full effect may not develop for 30 minutes. Oral administration is much less reliable and the tranquillizing effect is greatly influenced by whether the drug is administered with food (poor effect) or on an empty stomach (better effect).
An advantage to including acepromazine for premedication is that it provides some protection against catecholamine-induced ventricular irregular rhythms. Acepromazine should be omitted from premedication when severe blood loss or hypotension is anticipated during surgery as the peripheral α blockade complicates treatment of hypotension. Acepromazine is not recommended for use in brachycephalic breeds or dogs at risk for upper airway obstruction. Acepromazine has been avoided in the past in dogs with a history of seizures but there is recent evidence that administration of acepromazine to dogs with epilepsy has not initiated seizures (Tobias et al., 2006).
Other phenothiazine derivatives that are used include propionyl promazine, promazine, promethazine, methotrimeprazine, and chlorpromazine. The side effects produced by them and the provisions of use are similar to those of acepromazine.
Benzodiazepines
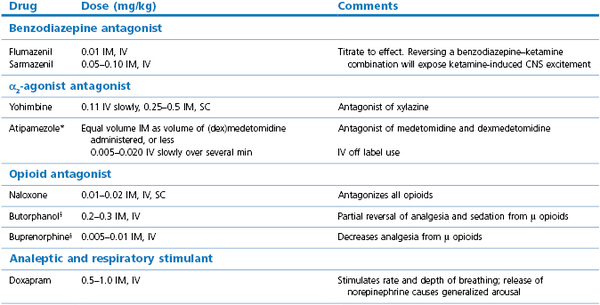
The benzodiazepines, for example, diazepam, midazolam, and climazolam, are frequently used in combinations with opioids, α2-agonist sedatives, and anaesthetic agents in veterinary practice to augment sedation, decrease dose rates of other agents, and to block excitatory effects of injectable anaesthetic agents. Benzodiazepines rarely are administered alone to healthy dogs because restlessness, agitation, or even excitement may follow. In contrast, sedation may be induced in geriatric dogs, ill dogs, and dogs with meningitis or seizures. Intravenous administration produces a more intense effect than IM. Diazepam is reserved for IV use as the formulation interferes with IM absorption whereas midazolam is water soluble and administered either IV or IM. The benzodiazepines induce minimal cardiovascular or respiratory effects and have a wide margin of safety. Thus they are good alternative agents to acepromazine in old or sick dogs. Benzodiazepines can be reversed by flumazenil or sarmazenil.
α2-Agonist sedatives
These sedatives are widely used in veterinary practice for their profound sedation and analgesic properties. They induce significant cardiorespiratory changes (see Chapter 5). The effects include CNS depression, hypnosis and analgesia, decreased cardiac output, increased blood pressure followed by a decrease, and bradycardia. Second degree atrioventricular (AV) heart block develops in some dogs. Since administration of moderate doses of medetomidine and dexmedetomidine decrease cardiac output to 21–40% of the preinjection value (Kuo & Keegan, 2004; Congdon et al., 2011), cautious use and decreased dose rates are recommended for sick dogs and those with cardiac disease. Ventilation and PaCO2 may not significantly change during medetomidine sedation but the respiratory centre sensitivity and response to CO2 is depressed and some dogs may become hypoxaemic (Kuo & Keegan, 2004; Lerche & Muir, 2004).
Peripheral vasoconstriction increases the difficulty of inserting an IV catheter and interferes with monitoring of patient oxygenation as the gums may be white and a pulse oximeter probe may not be able to detect a signal. Gastrointestinal motility is decreased. Bloody diarrhoea occasionally has occurred up to 24 hours after sedation with medetomidine and dexmedetomidine administered at the higher dose rates, perhaps caused by ischaemia. Decreases in ileal and colonic microvascular blood flows have been measured, presumably resulting from decreased cardiac output and increased systemic vascular resistance (Pypendop & Verstegen, 2000). This group of drugs also causes decreased serum insulin and drug-dependent varying increases in blood glucose concentration, and increased urine output.
The intensity of sedation from injection of these drugs can be influenced by the mental status of the dogs such that sedation is less when the dog is frightened or excited. Sudden arousal from apparently profound sedation with medetomidine may occur and has resulted in serious facial injuries in personnel leaning close to the dog, a reaction that may occur even in previously good-tempered dogs. Concurrent administration of an opioid decreases the likelihood of arousal. The addition of atropine or glycopyrrolate with medetomidine or dexmedetomidine to prevent bradycardia is not recommended because the increased heart rate (HR) may result in extreme hypertension and cardiac dysrhythmias (Ko et al., 2001b; Congdon et al., 2011).
Xylazine was the first α2-agonist sedative to be widely used in veterinary medicine. Xylazine, 0.5–2.2 mg/kg, IM will produce dose-dependent sedation and will markedly decrease dose rates of other anaesthetic agents administered subsequently. Most dogs will retch or vomit after administration of xylazine as sedation is developing.
There is evidence that xylazine sensitizes the myocardium to catecholamines, although the validity of the test has been questioned. If xylazine increases the prevalence of ventricular dysrhythmias that may explain the increased risk of death occurring during or after anaesthesia in which xylazine was part of the protocol (Dyson et al., 1998).
Medetomidine is a racemic mixture of two stereoisomers, dextro-medetomidine and levo-medetomidine. It is marketed for small animal practice as 1 mg/mL (1000 µg/mL). Medetomidine provides better sedation and analgesia than xylazine and has a longer duration of action (Tyner et al., 1997). The cardiopulmonary effects of medetomidine are similar to previously described, however, the changes are quantitatively similar for doses ≥5 µg/kg (Pypendop & Verstegen, 1998). Sedation is achieved at 2 µg/kg IV and increasing the dose rate increases the intensity and duration of sedation, from 60 minutes after 5 µg/kg to 120 minutes after 20 µg/kg. Vomiting occurs in about 20% of dogs receiving medetomidine, which is less than xylazine. Medetomidine, 0.01–0.02 mg/kg (10–20 µg/kg), IV significantly decreases serum insulin concentration but plasma glucose concentration remains within the normal physiological range (Burton et al., 1997).
Medetomidine has a steep dose–response curve and doses should, ideally, be calculated on a body surface area (BSA) rather than on body weight. In practice, this means that large dogs require relatively lower doses than smaller dogs. The IM dose rates given to induce profound sedation are up to 750–1000 µg/m2. The cut-off weight between higher and lower dose rates is 15 kg, so that dogs <15 kg need a higher dose and dogs >15 kg need less. The highest dose rate for a 15 kg dog is roughly equivalent to 0.03–0.04 mg/kg (30–40 µg/kg). The product information sheet contains detailed tables on volume of drug to administer for different weights of dogs. A formula for calculating BSA using kg bodyweight is BSA m2 = (Bwt kg2/3 × 10.1)100 (Pypendop & Verstegen, 2000; Hill & Scott, 2004). The sedative effect is increased in senior dogs and frequently half the dose rate will have the same effect as the full dose in a younger dog.
The effects and use of dexmedetomidine are similar to those described for medetomidine. The recommended dose rate for inducing mild sedation is 125 µg/m2, for moderate sedation 375 µg/m2, and for profound sedation 375–500 µg/m2. The manufacturer has marketed a solution (0.5 mg/mL, 500 µg/mL) that is half the concentration of small animal medetomidine to facilitate ease of transition from medetomidine to dexmedetomidine so that the volume to be injected is an identical volume for volume substitution. The product information sheet has detailed tables with volume/dose for dogs of varying body weights. In clinical practice, these dose rates must be modified based on evaluation of the individual patient and the required effect.
Romifidine has been administered IM or IV and induces sedation and bradycardia for 2–3 hours. The dogs may vomit before assuming recumbency. Cardiac output is decreased and central venous pressure (CVP) is increased (Pypendop & Verstegen, 2001). Romifidine ≥0.025 mg/kg (25 µg/kg) IV initially increases mean arterial pressure (MAP) followed by a prolonged decrease; MAP is immediately decreased by lower doses. Second degree AV block has been observed. Romifidine at 0.02 mg/kg (20 µg/kg) and 0.04 mg/kg (40 µg/kg) IM has been used alone or in conjunction with a low dose of anticholinergic agent (atropine, 0.01 mg/kg, or glycopyrrolate, 0.001 mg/kg) for sedation and for premedication to inhalation anaesthesia in experimental and clinical dogs (England & Hammond, 1997; Redondo et al., 1999; Lemke, 2001). The dose rate of the induction agent was significantly decreased, thiopental to a mean of 6.5 mg/kg and propofol to 3–4 mg/kg. Administration of an anticholinergic at the doses studied appeared to induce acceptable changes in HR and MAP and may have reduced the incidence of vomiting (Lemke, 2001).
Opioids
When administered alone, few opioids cause significant sedation in healthy dogs. When combined with a sedative or acepromazine, the result can vary from mild to heavy sedation depending on the route of administration, the drug and dose rate used, and the mental and physical status of the animal. Opioids can be used to provide analgesia for animals in pain, as part of an anaesthetic protocol for which a painful medical or surgical procedure is to be performed, and in combination with a sedative to deepen the intensity of sedation (see Table 15.1).
Panting is a side effect of opioid administration that may interfere with the procedure to be performed and may occur in as high as 70% of patients given morphine, oxymorphone, hydromorphone, or fentanyl, lasting from 30 minutes to 3 hours (Dohoo et al., 1986; Cullen et al., 1999). Vomiting is frequently observed after administration of morphine, oxymorphone, and hydromorphone (Box 15.1). Vomition is unlikely to occur after butorphanol, buprenorphine, fentanyl or methadone. Behaviour changes have been reported in a small number of dogs, such as increased whining after administration of methadone, vocalization after hydromorphone or butorphanol, and temporary aggression to owners or objects for 1 hour to 3 days after acepromazine–oxymorphone or fentanyl–droperidol (Dohoo et al., 1986; Ingvast-Larsson et al., 2010) (Trim, personal observations).
Tramadol
Tramadol is structurally related to codeine and exerts an analgesic effect through weak µ receptor agonist action and blocking of serotonin and norepinephrine uptake in the pain pathways of the spinal cord. Systemic availability is equivalent from IV and IM administration. There are no dosage recommendations for tramadol in dogs but published dose rates are 2 or 3 mg/kg and a redosing interval of 8 hours. Tramadol provides improved postoperative pain control when combined with a non-steroidal anti-inflammatory drug (NSAID). In one study, dogs scheduled for ovariohysterectomy were premedicated with atropine, 0.04 mg/kg, acepromazine, 0.05 mg/kg, and either tramadol, 3 mg/kg, or morphine 0.5 mg/kg, SC before induction of anaesthesia with thiopental and maintenance with halothane (Kongara et al., 2012). Dogs premedicated with acepromazine–tramadol required significantly higher concentrations of halothane for anaesthesia than dogs given acepromazine–morphine. Half of the dogs in each group were given boluses of fentanyl, 1 µg/kg, to treat responses to surgical stimulation. The dogs required additional doses of analgesic agent by one hour after anaesthesia, approximately 4 hours after premedication. This time corresponds to the usual time interval (3–4 hours) for redosing of morphine. In another investigation of postoperative analgesia after maxillectomy or mandibulectomy, tramadol or codeine or ketoprofen, all at 2 mg/kg SC, or combinations of these drugs were administered 30 minutes before the end of surgery (Martins et al., 2010). Tramadol and codeine were repeated at 8-hour intervals for the 24-hour monitoring period. Rescue analgesia was administered to some dogs in every group based on pain scores that included responses to pressure at the surgical site. Although not statistically significant, mean pain scores were lowest in dogs given ketoprofen with tramadol or codeine. Rescue analgesia denotes the administration of an analgesic agent during episodes of pain that are not controlled by a patient’s scheduled analgesic regimen.
Injection of tramadol, 4 mg/kg, IV in experimental dogs anaesthetized with sevoflurane caused no change in HR or cardiac output (Itami et al., 2011). Mean arterial pressure increased for 15 minutes and then returned to pre-injection value. Measurement of systemic vascular resistance revealed a mild but prolonged vasoconstriction.
Maropitant
Maropitant (Cerenia®) is licensed for use in the USA and Europe for prevention of motion sickness and acute vomiting in situations such as chemotherapy for dogs ≥16 weeks of age (Sedlacek et al., 2008). It is recommended as a once-daily administration at 1 mg/kg SC or 2 mg/kg orally. Absolute bioavailability is much higher after SC administration than oral, partly due to a hepatic first-pass effect after oral dosing (Benchaoui et al., 2007). Feeding status was found to have no significant effect on absorption of the drug. Renal clearance was negligible and no dosage adjustment was considered necessary for patients with renal disease.
Maropitant is an antagonist for neurokinin (NK1) receptors for which Substance P is the agonist. These receptors are present in the dorsal root ganglia and spinal cord dorsal horn, ascending projections to the brain, and in some visceral tissues (Boscan et al., 2011). Studies evaluating the analgesic effects of maropitant have yielded conflicting results. Working on the premise that maropitant may be an effective analgesic for visceral surgery, an experimental study simulated ovariectomy in dogs anaesthetized with sevoflurane (Boscan et al., 2011). Maropitant, 1 mg/kg, IV injected over 10 minutes followed by an infusion to maintain a constant blood concentration was documented significantly to decrease by 24% the concentration of sevoflurane required to prevent response to traction on an ovary. A study recently presented at a national meeting compared premedication with saline, morphine, 0.5 mg/kg, or maropitant, 1 mg/kg, SC prior to propofol–isoflurane anaesthesia for ovariohysterectomy (Marquez et al., 2011). A significantly lower concentration of isoflurane was used for removal of the ovaries and skin closure in dogs that received maropitant for premedication compared with none; sevoflurane concentrations for dogs given morphine were intermediate between these groups. Some dogs panted at the time of intense surgical stimulation, 67%, 38%, and 35% in premedication groups saline, morphine, or maropitant, respectively. This limited evidence suggests that maropitant provides some analgesia but investigations are needed to define its role in comparison with the available options.
Sedative–opioid combinations
There are many publications reporting the effects of combinations of a sedative or acepromazine and an opioid, using different dose rates, routes of administration, and timing of administrations. In most cases, the combination results in greater intensity and duration of sedation than is achieved by the individual drugs. Combinations that include medetomidine or dexmedetomidine generally induce the greatest sedation and decreased cardiac output. Because these α2-sedatives exert such profound sedation and cardiovascular effects, the dose rates of the opioids used in combination are often lower than those used in combination with acepromazine or a benzodiazepine. Other factors influencing the sedative and cardiovascular effects of these combinations are the relative dose rates and route of administration, and the mentation, age and physical status of the patient. To summarize, advantages of these combinations include:
•Increased sedation over that achieved by either agent alone
•With some combinations and in some patients, particularly old patients, sedation may be profound.
NSAIDs
The addition of an NSAID is valuable for management of perioperative inflammation and analgesia (doses in Chapter 5). High doses of NSAIDs, hypotension, hypovolaemia, and anaesthetic agents have the potential to alter renal perfusion and may increase the risk of adverse renal effects of NSAIDs (KuKanich et al., 2012). Consequently, general anaesthesia of dogs receiving NSAIDs should include fluid therapy and appropriate management of circulatory changes to avoid risk of NSAID-induced renal toxicity. Anaesthesia is not a contraindication to NSAID administration, nonetheless, some clinicians prefer to administer the NSAID at the end of anaesthesia when it is known that the dog is hydrated and not hypotensive. The effects of NSAIDs in dogs with renal disease have not been reported but cautious use in these patients is recommended (KuKanich et al., 2012).
Dipyrone (metamizole)
Dipyrone, also known as metamizole, was first produced commercially in Germany in 1922. The occurrence of agranulocytosis with some mortality in human patients led to failure of licensure of dipyrone in several countries, including the USA, the United Kingdom, Sweden, and Australia. The incidence of this adverse effect is low but the potential for the number of cases with widespread use is disputed. Dipyrone/metamizole is available by prescription in some countries and over the counter for self-medication in others. In some countries, dipyrone/metamizole has a veterinary licence for use in dogs and horses as an analgesic, antipyretic, antispasmodic, and (weak) anti-inflammatory drug. Inhibition of COX-3 in the brain and spinal cord is a proposed mechanism of action (Chandrasekharan et al., 2002).
Product information for dipyrone 50% in Canada states a dose rate in dogs of 50 mg/kg, to be administered IM or slowly IV at 8–12-hour intervals up to a maximum of 2 days. Several dose rates of metamizole have been evaluated for analgesic efficacy after ovariohysterectomy in dogs anaesthetized with acepromazine, propofol and isoflurane (Imagawa et al., 2011). Dogs given metamizole, 25 or 35 mg/kg IV 10 minutes before the end of surgery had significantly lower pain scores after surgery compared with dogs given tramadol. Four of 20 dogs given metamizole required rescue analgesia. Metamizole was repeated every 8 hours for 2 days. Vomiting in the first 6 hours after anaesthesia was observed in 40% of dogs and this effect was equally distributed through the treatment groups.
Antagonists
Specific antagonist agents are available for benzodiazepines (flumazenil and sarmazenil), α2-sedatives (atipamezole, and yohimbine and doxapram for xylazine), and opioids (naloxone) (Table 15.2). Flumazenil will reverse the effects of diazepam or midazolam overdose. Butorphanol will almost completely reverse the sedative effects of oxymorphone and hydromorphone (Dyson et al., 1990). Nalbuphine, 0.16 mg/kg, IV partially reversed oxymorphone but appeared to increase sedation when administered after buprenorphine (Jacobson et al., 1994).
General anaesthesia
None of the currently available anaesthetic agents have all the properties of an ideal agent. In clinical practice, one or more sedative, analgesic, or anaesthetic drugs are given before or during induction of anaesthesia to achieve safer anaesthesia by reducing the dose rates of individual agents. In fact, any of these agents may be suitable for most of our patients and the final choice may be determined by availability, familiarity of use or cost of the drug. However, in some patients, selection of one drug over another may increase safety of anaesthesia.
Preventive analgesia is aimed at attenuating the impact of all factors that stimulate pain pathways in the pre-, intra- and postoperative periods in order to reduce or prevent postsurgical peripheral and central sensitization and chronic pain-related behaviours. Preventive analgesia is demonstrated when postoperative pain and analgesic use are reduced beyond the duration of action of the agent used for analgesia (Katz et al., 2011). Although the surgical incision, wound retraction, and manipulation of organs may trigger central sensitization, so also can preanaesthetic noxious input and postoperative inflammatory mediators. Use of opioids alone is not sufficient, hence recommendations for including NSAIDs, nerve blocks, and other agents in the anaesthetic protocol. Patients with presurgical pain may not receive the same pain relief by analgesic therapy because central sensitization is already established. Even in healthy patients, preoperative factors such as genetic predisposition and psychological vulnerability may influence postanaesthetic management.
The intention of preanaesthetic evaluation is to avoid the development of complications. Unfortunately, complications frequently develop in patients not expected to have a problem with anaesthesia. All personnel involved in providing anaesthesia and related medical or surgical procedures should be familiar with the practice’s accepted plans for treatment of specific complications and resuscitation procedures. Plans should have been discussed before anaesthesia in a stress-free environment. Key points to a safer anaesthesia are listed (Box 15.2).