Chapter 13
Anaesthesia of sheep, goats, and other herbivores
Cornual nerve blocks for dehorning
Epidural and intrathecal nerve block
Femoral and sciatic nerve blocks
Peroneal and tibial nerve blocks
Intravenous regional analgesia
Intra-articular local analgesia
Ketamine and guaifenesin (glyceryl guaiacolate, GGE)
Potencies of isoflurane, sevoflurane, and desflurane
Non-steroidal anti-inflammatory agents
Local analgesia for castration
Sheep and Goats
Introduction
Anaesthetic management of sheep and goats is usually uncomplicated with the notable exception that regurgitation followed by pulmonary aspiration is always a risk of sedation and general anaesthesia. Tracheal intubation can be technically difficult, particularly in large rams and goats with large curled horns, because extension of the head and neck may be restricted and because the animals’ narrow jaws leave little room to view the larynx during insertion of the endotracheal tube. Two factors should be considered in selection of anaesthetic protocols for these ruminants. First, dose rates of some anaesthetic agents differ from those in other species. Secondly, animals that are not used to being handled or isolated may not exhibit signs commonly associated with sickness or pain. If these animals are sick they will have a reduced requirement for anaesthesia and failure to recognize this may result in overdosage.
This chapter describes techniques and drug combinations that can be used to anaesthetize sheep, goats, and camelids in a variety of settings. Local analgesia is useful in these species and details of the techniques are included. The anaesthetic agents listed in this chapter may not be available in all countries and many are not licensed for use in small ruminants. In some countries, ruminants given unapproved drugs cannot be used for food and, in others, some anaesthetic agents have approved withdrawal times. Current information on approved drugs, withdrawal times and extralabel drug use in North America has recently been published (Fajt, 2011). Choice of agents will also depend on whether the animal is intended for food, its health, procedure to be performed, and where the procedure will be performed (e.g. owner’s farm, veterinary clinic, or research facility). Some information on methods of anaesthesia of deer, camels, and elephants has been provided in the latter part of the chapter.
Accidental human injection of potent opioids, such as etorphine or carfentanil, α2-agonist sedatives, such as xylazine, detomidine and romifidine, and dissociative anaesthetics such as ketamine and tiletamine–zolazepam, can have serious consequences and even result in death (Haymerle et al., 2010). Preventative measures, such as wearing gloves and eye protection, particularly when working with darts, should be routine. Naloxone is the recommended antagonist for etorphine (25 ampoules should be available) and naltrexone is an alternative. Atipamezole is not licensed for use in humans at this time.
Local analgesia
Local analgesia techniques are highly useful in sheep and goat practice because equipment involved is inexpensive, cardiovascular and respiratory depression are less than produced by general anaesthesia, and the risk of regurgitation and aspiration is decreased. The immediate post-surgical recovery time may or may not be shorter than after general anaesthesia, depending on the agents and techniques used. Local anaesthetic agents may induce toxic symptoms when used inappropriately, especially when an excessive volume of local anaesthetic solution is injected. Limiting the initial administration of lidocaine to less than 6 mg/kg and bupivacaine to less than 2 mg/kg has been found to be safe. Bupivacaine is highly cardiotoxic and must never be injected IV. In Europe, at the time of writing (2012), the only agent with a marketing authorization for ruminants as food animals is procaine. In one investigation testing the effects of six local anaesthetic agents in ewes, cardiovascular toxicity in conscious animals was manifested initially as decreased contractility that was followed by seizures and tachycardia, progressing to ventricular tachycardia and, in some individuals, to cardiovascular collapse and death. Seven out of 36 sheep died after receiving slow IV administration of bupivacaine 2.1 mg/kg, levobupivacaine 2.6 mg/kg or ropivacaine 3.2 mg/kg (Copeland et al., 2008). None of the sheep given lidocaine or mepivacaine, 7.4 mg/kg, died. Anaesthetized sheep given these agents developed profound cardiovascular depression and all survived despite plasma concentrations of local anaesthetic twice that achieved in conscious animals.
Ropivacaine was introduced into the human medical market to offer a safer alternative to bupivacaine as the toxicity is lower (Leone et al., 2008). The toxicity of ropivacaine in herbivores has yet to be determined.
Options to extend the duration of epidural or spinal analgesia include addition of an opioid, such as morphine or buprenorphine, or an α2-adrenoceptor agonist, such as xylazine or dexmedetomidine. Controlled-release of bupivacaine and dexamethasone from polymer micropheres and liposomes is being investigated for prolonged (days) neural blockade (Dräger et al., 1998).
Applications of local anaesthesia
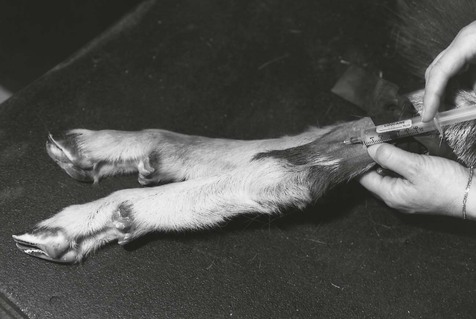
The cornual branches of the zygomaticotemporal (lacrimal) and infratrochlear nerves provide sensory innervation to the horns. The cornual branch of the lacrimal nerve emerges from the orbit caudal to the frontal process of the zygomatic bone (root of the supraorbital process). The nerve, covered by a thin layer of muscle, divides into several branches that innervate mainly the lateral and caudal parts of the horn. The main trunk of the infratrochlear nerve emerges from the orbit dorsomedially and divides into several branches. The cornual branch soon divides, one division coursing to the dorsal aspect of the base of the horn and ramifying dorsally and dorsomedially. The other division passes to the medial aspect of the base of the horn and gives off branches to the medial and caudomedial parts of it.
The site for producing block of the cornual branch of the lacrimal nerve is caudal to the frontal process of the zygomatic bone (root of the supraorbital process) (Fig. 13.1). The needle should be inserted as close as possible to the caudal ridge of the frontal process of the zygomatic bone to a depth of 1.0–1.5 cm in adult goats. The syringe plunger should be withdrawn before injection to check that the tip of the needle has not penetrated the large blood vessel located at this site. The site for blocking the cornual branch of the infratrochlear nerve is at the dorsomedial margin of the orbit (Fig. 13.1). In some animals, the nerve is palpable by applying thumbnail pressure and moving the skin over this area. The needle should be inserted as close as possible to the margin of the orbit and under the muscle to a depth of about 0.5 cm.
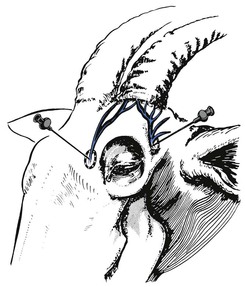
Figure 13.1 Nerve blocks for dehorning of goats. The corneal branches of both the lacrimal and infratrochlear nerves must be blocked. Care must be taken in young animals to ensure attempts to block both nerves do not lead to injection of toxic quantities of local anaesthetic solution.
Local analgesic solution such as 2% lidocaine, without epinephrine, should be injected at each site, about 2 mL/site for adult animals or up to a maximum of 6 mg/kg. Care must be taken with young animals not to exceed the toxic dose of lidocaine by limiting the total dose to 4 mg/kg, or inadvertently to inject local anaesthetic intravenously. Cornual nerve blocks are frequently combined with xylazine sedation, such as 0.05 mg/kg for adults and 0.025 mg/kg for kids. A subcutaneous line block with lidocaine may be required on the caudal border of mature horns.
Procaine is marketed in the UK as a 5% solution with epinephrine. In an evaluation of procaine for disbudding 99 dairy goat kids, all kids (median weight 5.3 kg) were injected with a total of 2 mL 5% procaine with epinephrine (12.2–28.6 mg/kg) as subcutaneous horn ring blocks (Pollard, 2008). At least 20 minutes were allowed before start of disbudding and analgesia was satisfactory for the procedure to be performed in the majority of animals. Muscle twitching and lethargy was observed in a few animals after the procedure but the cause was not confirmed.
Alternative analgesic techniques for dehorning include production of light general anaesthesia with diazepam or midazolam and ketamine, or other injectable or inhalation anaesthetic agents. It must be remembered when inhalation anaesthesia is employed, to discontinue oxygen and remove the facemask before application of a hot iron.
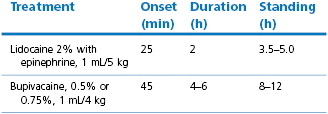
High epidural block can be produced by injection of local anaesthetic solution into the epidural space at the lumbosacral junction. Complete analgesia and paralysis can be induced in the hind limbs and abdomen to allow surgery, depending on the volume of local anaesthetic injected (Trim, 1989). The dose rates for different drugs and their times for onset of action are listed in Table 13.1. The dose rates listed are to produce analgesia for flank laparotomy. The dose should be decreased if the animal is old, obese, or pregnant. A lower dose of lidocaine, such as 2% at 1 mL 7 kg, is sufficient for perineal or hind limb surgical procedures, and for caesarian section. The long duration of hind limb paralysis from bupivacaine block for caesarian section interferes with nursing of the newborn and, for that reason, lidocaine with 1 : 200 000 epinephrine is preferred.
The intention behind inclusion of epinephrine with a local anaesthetic is to increase the intensity of sensory and motor block by decreasing uptake from the epidural space by local vasoconstriction. An added effect would be improved safety because the plasma concentrations of local anaesthetic agent would be lower. Addition of epinephrine has a significant impact on the duration of action of lidocaine, and has been documented to increase uptake of bupivacaine into cerebrospinal fluid (CSF), but has less effect on ropivacaine, presumably because ropivacaine has intrinsic vasoconstrictor properties (Ratajczak-Enselme et al., 2007).
The lumbosacral junction is easy to palpate in thin animals but recognition of landmarks will be necessary to identify the point of needle insertion in muscled or fat animals. Epidural block can be performed with the sheep or goat standing or in lateral recumbency. An imaginary line between the cranial borders of the ilium crosses between the spinous processes of the last lumbar vertebrae (Fig 13.2). The caudal borders of the ilium, where the angle bends to parallel midline, are level with the cranial edge of the sacrum. The point of needle insertion is midline halfway between the spinous process of the seventh lumbar vertebra and the sacrum. If the spinous process of the last lumbar vertebra can be palpated, the next depression caudal to it is the lumbosacral space. This area must be clipped and the skin prepared with a surgical scrub. A spinal needle should be used because it has a stilette to prevent injection of a core of subcutaneous tissue into the epidural space. The notch on the hub of the needle indicates the direction of the bevel and thus the anaesthetist can ensure that injection of local anaesthetic solution is towards the head of the animal.
Figure 13.2 Black pen has been used to identify the landmarks used to locate the lumbosacral space in a goat. An imaginary line between the cranial edge of the ilium crosses midline between the spinous processes of the last two lumbar vertebrae. The wings of the ilium angle obliquely towards midline and the sacrum (S).
When epidural nerve block is to be performed on the conscious animal, 1–3 mL 2% lidocaine should be injected subcutaneously with a fine needle at the site intended for insertion of the spinal needle. For lambs, kids, and pygmy goats, a 22 gauge 3.7 cm spinal needle can be used. For adult animals, a sturdier needle, such as a 20 or 18 gauge 6.25 cm spinal needle, is recommended. The needle should be inserted midline perpendicular to the curvature of the hindquarters and perpendicular to the midline sagittal plane of the animal, i.e. not necessarily parallel or perpendicular to the floor or table top (Fig. 13.3). If analgesia of one side or leg is required, the animal should be placed in lateral recumbency with the side to be desensitized underneath for the duration of the injection. Even though bilateral analgesia will develop, hyperbaric solutions such as bupivacaine will travel more cranially on the down side. After injection the spinal needle is withdrawn, the animal should be positioned so that the vertebral column is horizontal. If standing, the goat or sheep should not be allowed to progressively ‘dog-sit’, otherwise analgesia does not fully develop cranially.
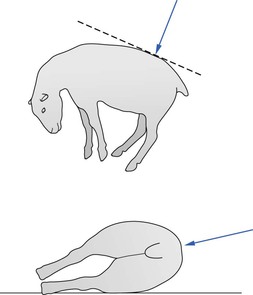
Figure 13.3 Direction of insertion of needle for lumbar epidural injection in sheep in lateral recumbency.
Considerable pressure may be needed to introduce the needle through the skin and supraspinous ligament and it may be preferable to puncture the skin first with a larger, sharp hypodermic needle. Once introduced, the spinal needle should be advanced gently for two reasons. First, to be able to appreciate the resistance then penetration of the interarcuate ligament which lies over the epidural space, described as a ‘pop’, and secondly, to control introduction of the tip of the needle into the epidural space so that movement of the needle can be stopped immediately. Further introduction of the needle will penetrate the spinal cord and the animal, if conscious, will jump and may dislodge the position of the needle. If the tip of the needle strikes bone and the needle does not appear to be deep enough to be in the epidural space, the needle should be withdrawn until the tip is just under the skin and redirected in a cranial direction. If unsuccessful, the procedure should be repeated with the needle advanced in a caudal direction.
After correct placement of the needle, the stilette should be removed and placed on a sterile surface. A 3 mL syringe containing a small volume of saline or air (up to 0.5 mL for large sheep/goats) should be attached to the spinal needle and the plunger withdrawn to test for CSF or blood. Attempts at aspiration should reveal only a vacuum and aspiration of air means that the syringe is not tightly attached to the needle. When no CSF or blood is aspirated, a test injection of a small amount of saline or air should be made (‘loss of resistance’ test). Injection should be easy when the needle is in the epidural space. After confirmation of correct placement of the needle, the 3 mL syringe should be detached and the syringe containing the local anaesthetic solution attached. Injection of the drug should be made over at least 30 seconds. Faster injections result in increased intracranial pressure which, if the animal is conscious, manifests as opisthotonus, nystagmus, and collapse.
Air has been used for many years for the ‘loss of resistance’ test of placement of a spinal needle in the epidural space. The injected air forms bubbles that interfere with even spread of the local anaesthetic solution, consequently, as small a volume as possible should be used. Use of saline or local anaesthetic solution (being mindful of the total dose injected) instead of air facilitates spread of subsequently injected local anaesthetic solution for nerve block.
A ‘hanging drop’ technique can be used to locate the epidural space when the animal is standing or positioned in sternal recumbency. The preparation of the skin, landmarks, and insertion of the spinal needle is the same as just described. The spinal needle is advanced until the tip is close to but not penetrating the interarcuate ligament. The stilette is removed and a drop of local anaesthetic solution or saline placed on the hub of the needle. When the needle is advanced through the interarcuate ligament, the drop of liquid will be sucked into the needle. This manoeuvre is used as confirmation of accurate placement of the needle in the epidural space, although failures may occur.
The spinal cord may project into the sacrum in sheep and goats and penetration of the dura will result in aspiration of CSF. Injection into the subarachnoid space of the same volume of local anaesthetic intended for epidural analgesia will result in the block extending further cranially and, potentially, respiratory arrest. Usually, the volume for subarachnoid injection is half the epidural dose. It is fairly common practice after accidental penetration of the subarachnoid space partly to withdraw the spinal needle and redirect it into the epidural space a few millimetres distant from the original insertion. Although initially CSF will leak into the epidural space because of the pressure difference, an experimental study in sheep where dural punctures were made one hour prior to epidural injection of morphine demonstrated that CSF concentrations of morphine were dramatically increased, even when the hole was made with a 25 gauge needle (Swenson et al., 1996). The concern with use of morphine was respiratory depression developing several hours later.
Entry of the needle into a venous sinus should be detected by aspiration of blood, an important manoeuvre as intravenous injection of local anaesthetic agent or morphine will result in cardiovascular depression. The local anaesthetic solution should be warmed when epidural injection is to be made in the conscious animal. Injection of a cold solution will stimulate receptors in the spinal cord and the animal will jerk, possibly dislodging the needle.
Catheters can be placed in the epidural or subarachnoid (intrathecal) spaces to facilitate administration of agents for surgical procedures and to continue regional analgesia postoperatively. Spinal catheters are available in a variety of materials and designs. The end of the catheter can be open or it can be closed with lateral holes (eyes) a few millimetres back from the tip. The opposite end can have an attached luer-lok adapter or an adjustable adapter that snaps onto the catheter tubing. Using sterile technique, a Tuohy needle (Fig. 13.4) is inserted at an angle through the skin at the lumbosacral junction until the tip is in the epidural space or advanced deeper to penetrate the meninges, allowing free flow of CSF when the stilette is removed. The catheter is inserted through the needle and advanced approximately 10 cm from the tip of the needle, whereupon the needle is removed carefully without dragging the catheter. An alternative technique is to insert a flexible guide wire to facilitate advancement of the catheter into the desired space (modified Seldinger technique). The needle is removed and the catheter threaded over the guide wire that is then removed. Extreme care must be taken to secure the catheter to the skin at the site of entry and to ensure sterile injections by covering the skin insertion with bandage and protecting the free catheter end – capped and wrapped in sterile gauze, for example.
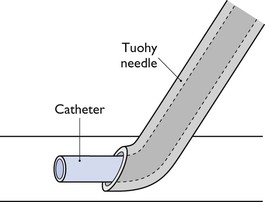
Figure 13.4 Catheter emerging from tip of Tuohy needle. The tip of a Tuohy needle is curved to aid bending of a catheter from the needle into the epidural or subarachnoid space.
Epidural and intrathecal analgesia are not totally without risk. The incidence of complications is probably very low but has not been documented in these species or other large animals (Trim, 2007). Potential complications include spinal haematoma, meningitis, or abscess associated with technique, and pruritus with morphine. Continuous infusions of opioids through intrathecal catheters are frequently used for long-term control of non-cancer pain in humans and there is increasing concern about the development of inflammatory masses adjacent to the tip of the catheters that compress the spinal cord and cause neurological deficits (Yaksh et al., 2002; Follett, 2003; Bedforth & Hardman, 2010). The inflammatory masses that develop in humans commonly do not manifest until after a year of use, however, experimental studies in dogs and sheep have identified inflammation, masses, and functional motor deficits occurring within 28–30 days of continuous infusion of morphine (Coombs et al., 1994; Gradert et al., 2003; Yaksh et al., 2003). The data are confusing at present. Morphine appears to be associated with inflammation, with the degree increasing with increasing dose and concentration and worse with continuous infusion compared with interrupted boluses twice daily. Hydromorphone has been associated with inflammation and, in one study (Johansen et al., 2004), even though inflammation was not greater than caused by saline injections, all the sheep exhibited gait deficits and biting behaviour over the dorsal lumbar area at the site of injection.
Placement of a venous catheter is a sensible precaution when epidural analgesia is to be used for surgery. Epidural injection of local anaesthetic solution causes paralysis of the sympathetic nerves and results in a decrease in blood pressure. Hypotension may develop, especially in hypovolaemic animals, such as for caesarian section, or animals positioned in such a way as to promote pooling of blood in the hind limbs. In these animals, treatment should include expansion of blood volume with intravenous administration of balanced electrolyte solution and injection of ephedrine, 0.06 mg/kg, to induce vasoconstriction. An animal with urethral obstruction given epidural analgesia for perineal urethrostomy or cystotomy may already have a distended urinary bladder at risk for rupture. Nonetheless, administration of fluid intravenously is important to restore blood volume and cardiovascular function. One option is to decrease the size of the bladder by cystocentesis. Alternatively, fluid infusion can begin at the time of epidural administration so that surgical relief of distension can be accomplished before a substantial increase in urine production occurs.
Approximately 50% of human patients experience visceral pain during caesarian section under epidural block with bupivacaine and describe the pain as poorly localized, dull pain, or as a feeling of heaviness or squeezing (Alahuhta et al., 1990). Sheep or goats may respond by movement or vocalization to manipulation of viscera during laparotomy under epidural analgesia with lidocaine or bupivacaine. The animals may be made more comfortable by IV administration of butorphanol, 0.1 mg/kg, diazepam or midazolam, 0.05 mg/kg, or xylazine, 0.02 mg/kg. Disadvantages of adjunct drug administration include respiratory depression, pharyngeal relaxation that may promote regurgitation and pulmonary aspiration, and depression of the lambs or kids delivered by caesarian section.
Sensory block may extend several dermatomes cranial to the level of motor block. Limb movement is possible even when the animal is sufficiently analgesic for surgery. During recovery from epidural block, the ability to move the hind limbs may develop before analgesia is lost, although the ability to stand may not return until long after analgesia is gone.
Respiratory paralysis can occur if local anaesthetic solution travels cranially to the neck. This will occur if too large a volume is injected, e.g. inaccurate calculation of dose. In this event, general anaesthesia must be quickly induced, the trachea intubated and controlled ventilation applied until the animal is able to breathe again.
During recovery from epidural block produced by local anaesthetic solutions, the animal should be allowed to recover quietly. It will be able to maintain sternal recumbency but there is potential for injury to the hind limbs if it makes uncoordinated attempts to rise.
Epidural injection of morphine, 0.1 mg/kg, at the lumbosacral junction will produce analgesia without paralysis. Preservative-free solution, 1 mg/mL, will not cause irritation of the meninges. This technique can be used to decrease requirement for inhalation anaesthetic agent and to provide analgesia postoperatively. In one study in goats after stifle surgery, it was observed that goats that were given epidural morphine vocalized less and were less likely to grind their teeth than goats that were not (Pablo, 1993). The duration of pain relief appears to be variable but provided relief for 6 hours after abdominal surgery (Hendrickson et al., 1996).
Intrathecal injection of 2% lidocaine, 2 mg/kg, with buprenorphine, 0.005 mg/kg, in tranquillized adult goats provided sufficient analgesia to perform a 30-minute orthopaedic stifle surgery, and duration of analgesia lasted longer postoperatively than in a comparison group of goats given lidocaine with xylazine, 0.05 mg/kg (Staffieri et al., 2009). The time from subarachnoid injection to start of surgery was on average 13 minutes and the goats were standing in approximately 90–100 minutes. Analgesia lasted for 3 hours after lidocaine–buprenorphine and 2 hours after lidocaine–xylazine, with a waning effect over 24 hours.
Xylazine has been used for epidural or intrathecal analgesia at dose rates ranging from 0.05 to 0.2 mg/kg or higher. Xylazine, 0.05 mg/kg, diluted with saline to 0.1 mL/kg or added to other agents, may be used for epidural analgesia as an adjunct to general anaesthesia for surgery. Systemic effects of sedation and decreased gastrointestinal motility may accompany epidural administration of xylazine in ruminants. A higher dose of xylazine, 0.4 mg/kg, has been injected at the lumbosacral junction of rams to produce analgesia for surgery involving lateral deviation of the penis (Aminkov & Hubenov, 1995). Analgesia extended to T5–T6 within 10 minutes and lasted for 120–140 minutes. Signs of systemic absorption were observed and hind limb motor block lasted an average of 224 minutes. In cattle, administration of atipamezole, 0.025 mg/kg, reversed sedation and flank analgesia after epidural block from xylazine, 0.05 mg/kg, but only epidural administration of atipamezole reversed the ataxia (Lee et al., 2003).
An investigation of epidural and intrathecal administrations of dexmedetomidine, approximate dose 0.0025 mg/kg, in sheep identified the potency of dexmedetomidine in the epidural space to be 10-fold higher than when administered in the intrathecal space (Eisenach et al., 1994). Both routes of administration resulted in rapid decreases in arterial pressure and the mechanism proposed was a direct effect on the spinal cord decreasing sympathetic nervous system outflow.
Injection of 2 (1–4) mL (0.5 mg/kg) of 2% lidocaine solution into the epidural canal through the sacrococcygeal space will provide caudal epidural analgesia for obstetrical procedures involving the vagina, vulva, and perineum. Xylazine, 0.07 mg/kg, may be added to the lidocaine for a prolonged effect up to 36 hours that would be useful in the treatment of vaginal prolapse after lambing (Scott, 1996). A smaller volume of local anaesthetic, 0.75–1.0 mL of 1% lidocaine, will provide analgesia for the docking of lamb’s tails. Strict attention must be paid to aseptic technique to avoid complications and several minutes must be allowed for analgesia to develop.
Frequently, the procedure is performed with the animal restrained in a standing position. The wool must be clipped from over the sacrum and the base of the tail and a surgical scrub applied to the skin. The site for needle placement is located by moving the tail up and down and palpating the most cranial point of articulation. A 20 gauge hypodermic needle is inserted midline approximately at a 45° angle to the curvature of the rump so that the tip of the needle enters the vertebral column and may even thread for a few millimetres cranially.
Continuous caudal block can be achieved by placement of an epidural catheter as described in the previous section. Local analgesic solution is injected through the catheter whenever the animal shows signs of returning sensation. Precautions against complications have already been described.
In sheep and goats, lumbar paravertebral nerve block is carried out using techniques similar to those employed in cattle. For incisions through the flank, the thirteenth thoracic and first three lumbar nerves are blocked. For each of these nerves, up to 5 mL of 1 or 2% lidocaine is used, divided and injected using a 20 gauge needle above and below the transverse ligament, up to a maximum total dose of 6 mg/kg lidocaine. Onset of analgesia may be as fast as 5 minutes. Duration of analgesia is an hour, or longer when lidocaine with epinephrine is used.
Flank laparotomy can be performed using local infiltration of lidocaine in an inverted L pattern 2–3 cm cranial and dorsal to the intended skin incision site. Blebs of lidocaine must be injected subcutaneously and deep in the abdominal muscle at approximately 1–1.5 cm intervals along the injection site. The maximum dose of lidocaine to be injected at one time is 6 mg/kg and dilution of a 2% solution to 1% solution may be necessary to provide sufficient volume for injection. The duration of analgesia is about 1.5 hours.
Digital nerves are easily blocked with lidocaine at the sites described for cattle. For total analgesia of the metacarpus and digits, lidocaine or lidocaine plus bupivacaine can be infiltrated subcutaneously circumferentially at the carpal–metacarpal junction. Analgesia lasting over 12 hours can be achieved by infiltration of 80% of a combination of lidocaine, 1 mg/kg, and bupivacaine, 0.5 mg/kg, mixed well in the same syringe.
Recent publications have described use of femoral and sciatic nerve blocks as adjunct analgesic techniques to general anaesthesia in sheep and goats (Adami et al., 2011; Wagner et al., 2011). The nerves were located by insertion of a 22 gauge nerve locator using a pulse width 0.5 ms, frequency 1–2 Hz, and an initial current of 2.0 mA. The sites for needle insertion are first clipped and cleaned with a presurgical preparation. With the animal in lateral recumbency with the surgical leg uppermost, the landmarks for the sciatic nerve are the greater trochanter of the femur and the ischiatic tuberosity. The needle is inserted on an imaginary line between these points at one-third of the distance from the greater trochanter and with the needle at a 60° angle to the skin with the tip of the needle directed towards the greater trochanter. Nerve location is identified when contraction of the quadriceps and extension of the hip and tarsus are observed when the current is reduced to <1.0 mA (generally 0.5 mA).
The femoral nerve is located in the area of the proximal medial thigh. This site can be accessed by abducting the surgical leg 90% and extending it or by turning the animal over into the opposite recumbency. The femoral artery is palpated as proximally as possible and the nerve locator needle inserted cranial to the artery and perpendicular to the skin. Contractions of the quadriceps and extension of the stifle at a current of 0.5 mA identifies the site for injection of bupivacaine.
Bupivacaine (0.5% or 0.75% solution) was injected at 0.5 mg/kg per site. The nerve blocks improved analgesia during surgery (mean anaesthesia time 70–85 minutes), however, the benefit postoperatively was limited to the first 24 hours in one study (Adami et al., 2011) and provided no clear benefit in the other (Wagner et al., 2011). Motor blockade of the limb was present during recovery from anaesthesia, lasting less than 4 hours in the sheep (Wagner et al., 2011) and for longer in the goats in which anxiety and abnormal behaviour were observed (Adami et al., 2011).
Analgesia of the hind limb below the hock can be achieved by peroneal and tibial nerve blocks. The peroneal nerve is blocked by injection of 5 mL 2% lidocaine (in adult sheep or goats) where the nerve runs obliquely caudodorsally to cranioventrally approximately 2.5 cm below the lateral condyle of the tibia. The nerve can often be palpated by using thumbnail pressure to move the skin and underlying tissues. Analgesia of the dorsum of the foot is obvious from the animal’s stance (Fig. 13.5).
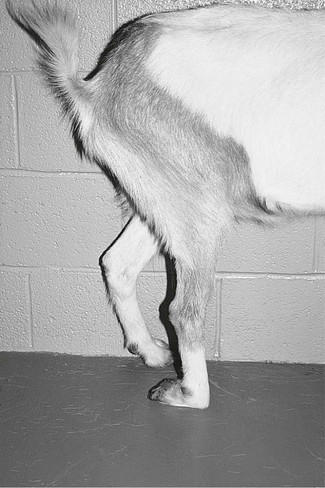
Figure 13.5 Peroneal and tibial nerve blocks produce analgesia distal to the hock. The hock will straighten and the animal will stand on the dorsum of the fetlock.
The tibial nerve is blocked by infiltration of 4 mL of 2% lidocaine solution on the medial side of the leg at the hock between the flexor tendons and the gastrocnemius tendon. A further 1 mL is injected at a similar site on the lateral side of the limb to block a small cutaneous nerve, a branch of the common peroneal nerve originating at the middle of the thigh. Onset of analgesia should be within 15 minutes and is accompanied by straightening of the hock (see Fig. 13.5).
Surgery on the limbs can be performed using intravenous regional analgesia (also known as a Bier block). In sheep and goats, the tourniquet or sphygmomanometer cuff is usually placed on the forelimb above the elbow (taking care not to pinch skin in the axilla) and on the hind limb above the hock (leaving sufficient length of saphenous vein for injection) (Fig. 13.6). The tourniquet must be sufficiently tight to block arterial flow without being excessively tight. The animal is less likely to react adversely to a flat band tourniquet than a round rubber tube. If using a sphygmomanometer cuff, it must be inflated to above systolic blood pressure. Injection of 4 mg/kg lidocaine without epinephrine should be made slowly through a 25 gauge needle in the cephalic or saphenous vein directed towards the foot. Care must be taken to keep the needle immobile within the vein during injection. Blood should be aspirated before injection to confirm needle placement within the vein. Onset of action is in 15–20 minutes. Analgesia will persist as long as the tourniquet is in place but sensation will rapidly return after the tourniquet is removed. The tourniquet should not be released within 10 minutes of the initial injection to allow time for the lidocaine to diffuse into tissues. Thereafter, releasing the tourniquet has no clinical effect on the animal. No long lasting effect has been noted in sheep or goats when the tourniquet has been in place for 2 hours.
The efficacy of intra-articular injection of local anaesthetic solution will depend on the nature of the surgery (because analgesia is limited to intra-articular structures) and the timing of administration. Intra-articular injection of lidocaine, 40 mg, before arthrotomy in adult sheep and another injection of bupivacaine, 10 mg, after joint closure were associated with significantly lower pain scores 3 to 7 hours postoperatively compared with sheep not receiving intra-articular analgesia (Shafford et al., 2004). Intra-articular injection of 0.75 mg/kg of bupivacaine in anaesthetized goats 30 minutes before the start of surgery significantly modified the increases in heart rates and mean arterial pressure (MAP) that occurred in response to stifle manipulation compared with animals injected with saline (Krohm et al., 2011). However, the level of analgesia provided by either pre- or post-surgical injection of bupivacaine was considered insufficient to eliminate the need for systemic analgesia. In experimental animals, 0.5% bupivacaine was found to inhibit synthesis of articular cartilage and cause inflammation whereas exposure to 0.125% bupivacaine was indistinguishable from saline (Webb & Ghosh, 2009). Lesser effects were produced by lidocaine and ropivacaine. Chondrolysis is an uncommon condition in humans that appears to develop after continuous administration of bupivacaine using a ‘pain pump’ and not from a single injection. The potential for occurrence and significance in other species is yet to be determined.
There has been considerable published research on analgesia in sheep for castration, tail docking, and mulesing (surgical removal of folds of perineal skin to minimize risk of fly strike). Analgesia may not be required in some countries for castration of very young ruminants. However, one study measuring EEG in lambs anaesthetized with halothane only for rubber-ring castration, identified a relatively low responsiveness of the cerebral cortex in the first few days of life with a progressive increase over the first 10 days of life (Johnson et al., 2009). The supposition was that this would represent the change in response to a noxious stimulus perceived at different ages. Behaviour changes in 5-, 21-, and 42-day-old lambs after castration and increases in plasma cortisol concentrations are all indicative of considerable pain after castration in the absence of analgesia (Molony et al., 1993). Considerable effort has been expended in the investigation of effective and practical analgesia for mulesing (Paull et al., 2007, Colditz et al., 2009). While not yet defined, significant reduction in indicators of pain associated with mulesing has been produced by use of topical local anaesthetic and injectable carprofen.
Peribulbar injection of lidocaine, approximately 1 mg/kg, or bupivacaine, approximately 0.25 mg/kg, is a useful adjunct to general anaesthesia for eye enucleation. The artery at the medial canthus is easy to lacerate, therefore, insertion of the needle at the lateral canthus and mid-dorsal and ventral edges of the orbit is preferred, and the total volume of local anaesthetic divided between the three sites. Injection is made with a 22 gauge spinal needle (needle point is less sharp than a hypodermic needle) bent to form a mild curvature to mimic the curvature of the globe within the orbit. It is important to deposit local anaesthetic between the orbit and extraocular muscles and to avoid penetration of the globe. Thus, the needle is inserted adjacent to the edge of the orbit and directed in a curve close to the bone until the tip is deep within the orbit. The syringe must be aspirated before injection to ensure that the needle is not in a blood vessel. The needle should be removed with the same curving action used for insertion, i.e. not in a straight line.
Sedation
Sedative agents employed in sheep and goats
Different sedatives and sedative–opioid combinations, and even low doses of anaesthetic agents such as ketamine, can be chosen to provide the desired characteristics for sedation in a particular situation. Dose rates are listed in Table 13.2. Descriptions and dose rates of drug combinations are in the text.
Table 13.2
Anaesthetic agent dose rates for sheep and goats*
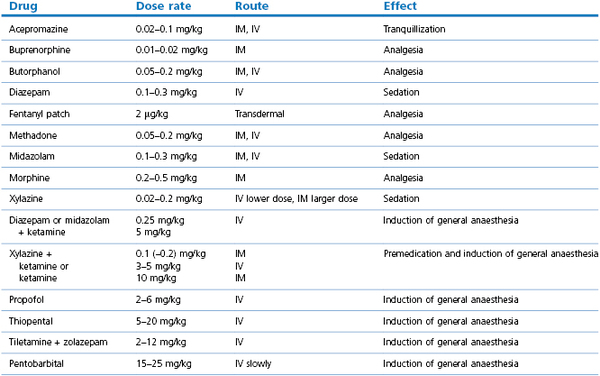
* Taylor (1998), Abu Serriah et al. (2007), Schauvliege et al. (2006), Ahern et al. (2009), Raske et al. (2010), Krohm et al. (2011), Wagner et al. (2011)
Preanaesthetic medication decreases the dose rate of induction and maintenance agents, calms the animal for induction, and provides a more stable anaesthesia.
Acepromazine, 0.05–0.1 mg/kg, can be used to provide mild tranquilization in sheep and goats. Since acepromazine induces vasodilation, this agent should be used cautiously in animals that are dehydrated or hypovolaemic.
Xylazine, detomidine, medetomidine, dexmedetomidine, or romifidine provide light to heavy sedation according to the drug, dose rate, and route of administration. The dose ranges are different than used in some other species because ruminants are more sensitive to these agents. Variation in the analgesic effects of xylazine has been noted in different breeds of sheep, for example, analgesia after xylazine was less in Welsh mountain sheep than in Clun sheep (Ley et al., 1990). The authors hypothesized that the difference may have been the result of dosing according to body weight rather than body surface area, but other factors such as different body compositions or temperament may have been involved. Animals that are young or sick will require only a low dose to induce sedation. The degree of sedation obtained from an α2-agonist sedative can be intensified by combination with a benzodiazepine or an opioid or ketamine. α2-Agonists can be used alone or in combination to provide satisfactory sedation for restraint or they can be used for premedication prior to induction of anaesthesia with other anaesthetic agents.
This group of sedatives induces marked physiological changes. A comparison of IV administration of xylazine, 0.15 mg/kg, detomidine, 0.03 mg/kg, medetomidine, 0.01 mg/kg, and romifidine, 0.05 mg/kg, to sheep maintained in an upright position indicated that all drugs caused a significant decrease in PaO2 (hypoxaemia) for 45 minutes that outlasted the duration of sedation, despite increased respiratory rates with no significant alteration in PaCO2 (Celly et al., 1997). Thus, hypoxaemia could not be attributed to hypoventilation or to a change in body position. Route of administration may influence pharmacological effects (IM less than IV) as medetomidine, 0.015 mg/kg, injected IV in sheep resulted in respiratory depression and hypoxaemia (Raekallio et al., 1998) whereas medetomidine, 0.03 mg/kg, administered IM induced a significant decrease in PaO2 but hypoxaemia in only one out of nine sheep (Kästner et al., 2003). The duration of sedation was longer after IM administration, with peak sedation occurring at 30–40 minutes and the time to return of normal behaviour exceeded 6 hours. Investigations of the pulmonary changes induced by xylazine or dexmedetomidine describe diffuse hyperaemia, severe pulmonary capillary congestion progressing to extravasation of red blood cells into alveoli and moderate to severe proteinaceous alveolar oedema at 10 minutes after IV administration (Celly et al., 1999; Kästner et al., 2007). One study design incriminated a hydrostatic cause of the pulmonary oedema associated with a two- to threefold increase in pulmonary artery pressure (Kästner et al., 2007). Xylazine administration has occasionally been associated with the development of clinical signs of pulmonary oedema in sheep.
Xylazine induces a short-lived decrease in heart rate and a mild decrease in MAP. Detomidine, medetomidine and romifidine induce significant bradycardia with a transient (10 minutes) increase in MAP (Celly et al., 1997). Comparison of the effects of dexmedetomidine, 2 µg/kg, administered IV in sevoflurane-anaesthetized sheep and goats revealed similar changes although there was considerable variation between individual animals in haemodynamic response (Kutter et al., 2006). Cardiac output and heart rate significantly decreased, MAP and systemic vascular resistance (SVR) initially increased followed by a decrease, and MAP and central venous pressure (CVP) increased. Heart rates decreased and CVP increased more in goats than in sheep. Thus, differences in pharmacological effect between individuals are unpredictable and there may be a variation in response between anaesthetized and conscious animals. No doubt the impact of these cardiovascular changes will depend on the dose rate of the agent, concurrent administration of other anaesthetics, and the physical status of the patient. The use and adverse effects of α2-agonists in sheep have been reviewed (Kästner, 2006).
α2-Agonist sedatives cause decreased intestinal motility and frequently result in ruminal bloat. Urine production is increased and care must be taken when administering these agents to animals with urethral obstruction. Xylazine is not recommended for use in the last trimester of pregnancy to avoid increasing contractility of the myometrium. Blood glucose increases after administration but to a lesser extent than in cattle.
Antagonists of the effects of α2-sedatives include tolazoline, up to 2 mg/kg, or doxapram, 0.5 mg/kg, for xylazine, and atipamezole, 25–50 µg/kg, for all the agents. The antagonists can be administered IV (slowly in increments), IM, or half IV and half IM.
Intravenous administration of diazepam, 0.10–0.25 mg/kg, or midazolam, 0.10–0.25 mg/kg, may produce some sedation, ataxia, and analgesia in sheep and goats for 15–30 minutes. The degree of sedation is unpredictable in healthy animals and, in some animals, these agents may induce excitement (Kyles et al., 1995; Raekallio et al., 1998). Midazolam IV or IM and diazepam IV will significantly increase the intensity of sedation when combined with another sedative or opioid. Respiratory depression will be increased and, when combined with medetomidine, haemoglobin O2 desaturation will be worse (Raekallio et al., 1998). The combination of midazolam, 0.25 mg/kg, and butorphanol, 0.1 mg/kg, IM provides good sedation in sheep for minor procedures such as radiography or cast removal.
Diazepam has been incriminated in the development of pulmonary oedema in a sheep (Stegmann, 2000). A 150% increase in pulmonary vascular resistance was measured in experimental sheep 3 minutes after IV injection of diazepam (Pearl & Rice, 1989). The pulmonary vascular changes have been attributed to propylene glycol induced release of thromboxane A2 (Quinn et al., 1990).
Flumazenil, 0.01–0.02 mg/kg, can be used to attenuate or reverse sedation and analgesia induced by benzodiazepines (Kyles et al., 1995). Sarmazenil is an antagonist of diazepam and midazolam available in Europe.
A combination of diazepam, 0.25 mg/kg, and ketamine, 5 mg/kg, can be mixed in the same syringe and administered IV in small increments to achieve the desired level of sedation for minor procedures.
Butorphanol, 0.05–0.20 mg/kg, IM or IV is useful to increase sedation from acepromazine, α2-agonists, or benzodiazepines. Butorphanol has a rapid onset of action and given 5–10 minutes before diazepam or midazolam and ketamine facilitates a smooth and relaxed induction of anaesthesia. The duration of effect appears to be 1–2 hours. The analgesic effects of butorphanol are not great and vary with the type of stimulus. For example, butorphanol is considered to provide unsatisfactory analgesia for orthopaedic surgery.
A variety of opioids have been administered to sheep and goats to provide analgesia. Meperidine (pethidine), 2–4 mg/kg, IM has been used for many years as an adjunct to anaesthesia in sheep and goats. Methadone, 0.05–0.5 mg/kg, in combination with midazolam, 0.3 mg/kg, IM or IV has been used for premedication to general anaesthesia in sheep and goats (Schauvliege et al., 2006; Adami et al., 2011; Krohm et al., 2011). Morphine is commonly administered at a rate of 0.2–0.5 mg/kg (Wagner et al., 2011). A higher dose of morphine, 2 mg/kg, IV during anaesthesia in experimental goats decreased the isoflurane requirement for claw clamping by 30%, however, the cardiovascular effects were not reported (Doherty et al., 2004).
The value of buprenorphine as an analgesic agent in small ruminants is in question. Clinical experience has shown that, in healthy sheep and goats, buprenorphine, 0.01 mg/kg, IM administered as a premedication agent before induction of anaesthesia appears to cause no sedation and minimal reduction in the isoflurane concentration required to prevent response to a surgical procedure. In contrast, administration of buprenorphine, 0.01 mg/kg, postoperatively appears to alleviate pain as judged by behaviour change, such as muscle relaxation, cessation of teeth grinding, and reversal of increased respiratory and heart rates. Buprenorphine is better given by the IM rather than IV route when administered alone as premedication in healthy animals because IV administration may induce excitement or restlessness. Despite pharmacokinetic studies that have shown that the terminal half-life of buprenorphine in goats and sheep is shorter than in cats and much shorter than in dogs (Ingvast-Larsson et al., 2007), buprenorphine, 0.01 mg/kg, IM is typically administered 45 minutes before anaesthesia and subsequently at 3–6 hour intervals. Buprenorphine given alone to healthy animals may induce agitation, however, tolerance develops after the first injection such that the animals are calmer after the second and subsequent injections (Ingvast-Larsson et al., 2007).
Experimental tests for analgesia are difficult to translate to clinical practice. No antinociceptive activity was detected for approximately 45 minutes in sheep following IV administration of buprenorphine, 0.006 mg/kg (Waterman et al., 1991). Buprenorphine, 0.0015 mg/kg, provided analgesia to the thermal threshold test for 3.5 h but neither this dose nor 0.012 mg/kg produced analgesia to a mechanical threshold test. Pharmacokinetic data confirmed that the drug was rapidly distributed in the body and that there was no correlation between plasma concentration and analgesic activity. In these animals, IV injection of buprenorphine produced excitement exhibited by rapid head movements and the sheep chewing on objects. In another study (Grant et al., 1996), sheep were tested with an electrical current and no analgesia was detected after administration of buprenorphine, 0.005 mg/kg, methadone, 0.6 mg/kg, or flunixin meglumine, 2.2 mg/kg, IM. Analgesia was detected after administration of xylazine, 0.05–0.2 mg/kg, in a dose-dependent manner using the same testing device. Another investigation in sheep compared fentanyl, 2 µg/kg/h applied as transdermal patches 12 hours before anaesthesia with buprenorphine, 0.01 mg/kg, administered at induction and IM every 6 hours to sheep undergoing tibial osteotomy (Ahern et al., 2009). There were no significant differences between the induction doses of thiopental and lidocaine or the isoflurane vaporizer percentages used. The postoperative pain scores for sheep receiving fentanyl were significantly lower than those of sheep receiving buprenorphine, but the scores gave no indication that rescue analgesia was required.
Etorphine and carfentanil are potent opioids that generally are used for immobilization of non-domestic animals. A study comparing IM administered etorphine or carfentanil, 10, 20, and 40 µg/kg of body weight, in instrumented goats described similar effects for both drugs (Heard et al., 1996). The goats were rapidly immobilized, more quickly with carfentanil (≤ 5 minutes) than with etorphine (5–10 minutes) and etorphine always induced transient struggling. Immobilization was characterized by limb and neck hyperextension with occasional vocalization and bruxation. The goats were partially recovered by an hour after etorphine administration but were unable to stand at 2 hours after carfentanil. Both drugs significantly increased blood pressure and decreased heart rates without changing cardiac outputs. Arterial O2 content was not decreased and the goats did not regurgitate.
General anaesthesia
Preparation
Withholding food and water from small ruminants before anaesthesia is not a universal practice. However, many prefer to withhold food for 24 hours and water for 0–12 hours before anaesthesia whenever possible to decrease pressure of the rumen on the diaphragm and aid ventilation, to decrease the severity of bloat, and to decrease the prevalence and volume of regurgitation. Lambs and kids should be prevented from nursing for 30–60 minutes before anaesthesia. When heavy sedation or general anaesthesia is to be administered to an unfasted ruminant, rapid-sequence induction of anaesthesia and intubation of the trachea should be performed.
It is doubtful if atropine has any value as a general premedicant in sheep and goats. The doses necessary to prevent salivation completely (0.2–0.8 mg/kg) produce undesirable tachycardia and ocular effects, while smaller doses merely make the saliva more viscid and hence more difficult to drain from the oropharynx. Furthermore, atropine may decrease intestinal motility in ruminants for hours or days, predisposing them to bloat and delaying return to eating. Bradycardia seldom develops during anaesthesia, except when an α2-adrenoceptor agonist sedative has been administered. Severe bradycardia can be treated by administration of atropine, 0.02 mg/kg, or glycopyrrolate, 0.005 mg/kg, IV. These dose rates may be inadequate and repeat doses may be required depending on the cause of bradycardia. Atropine up to 0.3 mg/kg has been used to treat bradycardia in sheep sedated with medetomidine (Clarke, personal communication).
Premedication is not essential before induction of general anaesthesia with injectable agents in small ruminants, as excitement at induction is uncommon. However, premedication with an opioid and sedative, either before or at the time of induction of anaesthesia provides muscle relaxation and analgesia, and decreases the dose rate of subsequently administered anaesthetic agents. Administration of an opioid, such as butorphanol, 0.05–0.20 mg/kg, IM or IV or buprenorphine, 0.01 mg/kg, IM will improve muscle relaxation and provide some analgesia. Other opioids, as previously described, may be selected to provide analgesia depending on the procedure to be performed.
Anaesthetic techniques
There are many sites available for venepuncture in sheep and goats and the choice depends on the site of the surgical procedure and the personal preference of the anaesthetist. The jugular vein is a common site for placement of a catheter. Adult sheep and goats are restrained standing with their heads bent away from the side of catheter insertion. Smaller animals can be restrained in lateral recumbency. The wool or hair must be clipped and surgical preparation of the skin performed before insertion of a catheter. A subcutaneous bleb of lidocaine at the site of catheter insertion will decrease the likelihood of the animal moving in response to the procedure. The vein should be occluded at the base of the neck, and the catheter (18 or 14 gauge, 7.6–13.3 cm long for mature animals) inserted into the jugular vein directed towards the heart. The catheter should be capped, flushed with heparinized saline, and sutured securely to the skin.
The cephalic vein in the forelimb and the saphenous vein in the hind limb are easily viewed after the wool or hair over them has been clipped. It should be noted that the cephalic vein is more oblique on the limb than in the dog (Fig. 13.7). Sheep can be effectively restrained by lifting them into a sitting upright position (Fig. 13.8). The sheep can be tilted slightly to one side to facilitate access to the saphenous vein and the position conveniently extends the hind limb for catheter placement. Goats are better placed in sternal or lateral recumbency for venepuncture of the cephalic or saphenous veins. A catheter (18 gauge, 5 cm long) can be inserted into either vein, capped, flushed, and secured to the leg with adhesive tape around the catheter and around the leg. A Butterfly needle (21 or 19 gauge) can be used in the cephalic vein.
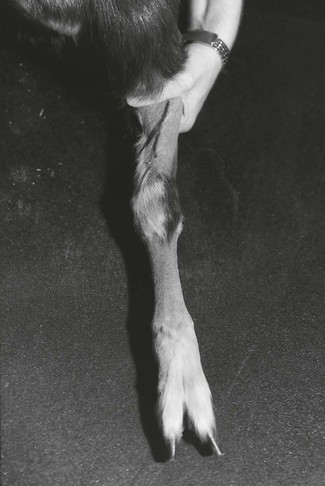
Figure 13.7 The cephalic vein in a goat is short and oblique across the forearm (black pen has been used to identify the location on the left cephalic vein in this animal).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree