Chapter 30 Amphibian Viral Diseases
In earlier editions of this text, viral infections of amphibians received little consideration because they were not considered to be of great clinical importance or were of purely historical significance. In the intervening years, amphibian viruses have been demonstrated to be primary pathogens in both anurans and urodeles. They have been incriminated in morbidity and mortality in wild amphibian populations around the world and likely play a role in the natural ecology of a species, although without having the devastating long-term impact of fungi. Conversely, there have been few reports of viral infections in zoo collections.7,15 Outbreaks of viral disease in captive animals have principally been reported from commercial frog farms in which the conditions may more closely resemble those in the wild than in the more controlled conditions of zoo collections.14,16 Excellent papers and reviews on viral infections have been published, but this is a rapidly expanding field.4,6,9,12,13
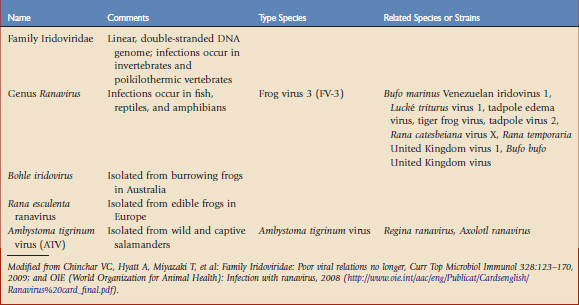
The most significant and well-studied amphibian viruses belong to the family Iridoviridae (Table 30-1). The taxonomic status of this group is fluid and may be confusing. Iridoviruses have only been isolated from poikilothermic animals and are usually associated with damp or aquatic environments. The host range is variable and there is evidence of transmission across orders or even phyla. The family is currently divided into five genera and several unclassified members. Two genera, Iridovirus and Chloriridovirus, infect invertebrates such as insects, crustaceans, and mollusks. Lymphocystivirus and Megalocystivirus infect fish. Those infecting amphibians belong to the genus Ranavirus. The term iridovirus may denote either a member of the family Iridoviridae or a member of the genus Iridovirus.3
Ranaviruses
Ranaviruses are large (120 to 300 nm), icosahedral, linear, double-stranded DNA viruses. Virus particles may be enveloped (obtained from the plasma membrane) or unenveloped, with viral replication occurring in the cytoplasm or nucleus. Within the genus Ranavirus, species and tentative species are named based on the type of disease they induce, the animal from which they were first isolated, or their site of origin.3
Ranaviruses have been identified in an increasing number of wild amphibian hosts around the world. The original frog isolates were identified in a study of renal carcinomas in leopard frogs (Rana pipiens) but were not associated with any lesions at that time. However, disease outbreaks among fish, cultured and wild frogs, and salamanders have since implicated several members of the genus Ranavirus as pathogens.3 There are limited reports of infections in zoo or pet animals but a survey of wild-caught frogs in a zoo collection has shown a high prevalence of ranavirus based on diagnosis by polymerase chain reaction (PCR) assay in live and dead animals.7 Infection also occurs concurrently with other pathogens, including bacteria and fungi.15 It is not known whether the paucity of reported cases of infection in captive animals reflects a low viral prevalence or virulence, or the fact that ranavirus infections are not recognized because the clinical and pathologic findings are often nonspecific.20
Ranavirus infections are not limited to a single species or taxonomic class of animal. Each virus or viral strain shows a slightly different genomic structure or molecular fingerprint, depending on the location of the isolate or the species in which it was found. Isolation of a ranavirus from a new species of amphibian does not necessarily identify new viral species. At the time of this writing, there are several distinct type species affecting amphibians: frog virus 3 (FV-3), Ambystoma tigrinum virus (ATV), Bohle virus, and Rana esculenta virus (REIV).3,17
ATV was isolated from tiger salamanders (Ambystoma tigrinum). Other species closely related to ATV, such as Regina ranavirus, have been identified in other urodeles, and Axolotl ranavirus was found in a laboratory collection of axolotls (Ambystoma mexicanum). In experimental studies, anurans are variably, but generally less, susceptible to infection with ATV-like viruses than urodeles.24 Bohle iridovirus, a virulent pathogen of the burrowing frog Lymnodynastes ornatus in Australia, may be experimentally transmitted to fish and marine toads.
Clinical Effects
Although many of these viruses cause life-threatening infections, subclinical infections also occur.3,21 Ranaviruses may be transmitted experimentally by injection or by immersion in infected water, and naturally by cohabitation, predation, and wounding. In anurans, viruses such as FV-3 are associated with systemic disease manifested by two major syndromes; the most severe manifestation is one of hemorrhage and edema in internal organs and, in the less acute form, affected animals show erythema, ulceration, or hyperplasia of the skin.1,5,6 In experimentally infected frogs, those developing the hemorrhagic syndrome died in the acute or peracute stages of the disease within 1 to 2 weeks of infection, whereas frogs with the ulcerative form developed lesions more slowly. These differences may be manifestations of degrees of severity influenced by variations in pathogenicity of the strain of the virus, immunity of the amphibian, route of infection, or weight of viral challenge.
Signs in salamanders are similar. Early lesions include white polyps on the skin of the epidermis that progress to cover most of the body, with progressive hemorrhage and ulceration. The skin may become dark or speckled. Affected animals become lethargic, refuse food, and float near the surface of the water, finally developing loose bloody feces, emesis, anorexia, edema, and cutaneous erosions and ulcers. Death may occur within 48 hours of developing bloody feces.1,11,19 Progression of the disease may be affected by the ambient temperature. In some experiments, mortality was reduced at elevated temperature, perhaps a result of enhanced immune function; in others, viral replication itself was temperature-dependent.22
These syndromes are consistent with descriptions of red-leg, which historically has been regarded as having a primary bacterial (usually Aeromonas. hydrophila) cause. Although various bacteria may indeed be responsible, ranaviruses have also been shown to cause syndromes in which neither the outcome nor progression of the disease was altered by the presence of bacteria, including A. hydrophila.5,6
Late-stage tadpoles and metamorphs are generally the most susceptible to infection, whereas adults are less frequently affected, a difference likely related to viral virulence and innate or acquired host resistance factors. The high susceptibility of larval stages to ranaviruses has been established in Xenopus spp.; however, normal adults of this species are resistant to infection.21 In other cases, tadpoles as young as 2 weeks of age died while very young tadpoles or those nearing metamorphosis were unaffected.14 Typically, diseased tadpoles stop feeding, remain at the bottom of a tank, swim abnormally, and appear deformed. Abdominal distension is common because of fluid retention and generalized edema, perhaps reflecting the predilection of the virus for the kidneys. Skin lesions or petechiae may not always be present. Mass mortalities of tadpoles may be seen, with up to 100% of a tank population lost.
Pathologic Findings
Pathologic findings in ranaviral infections mirror the clinical syndromes. FV-3 targets the renal proximal tubular epithelium and erythropoietic tissues, notably in the liver and spleen, and also affects other tissues.* Frogs dying of the hemorrhagic syndrome show an enlarged congested spleen. Petechial or diffuse hemorrhage may be seen on the urinary bladder, testes, intestines, and other viscera. Hemorrhages may also be seen on the skin and in the mouth subcutaneously and in the musculature of the legs, most notably of the hindfeet. Chronic skin ulceration in the absence of internal lesions has also been described. In salamanders, the coelomic cavity often contains clear fluid, with petechiation on the serosal surfaces, edema and hemorrhage in the wall of the stomach, and a mottled liver. Histologically, infected frogs and tadpoles show hemorrhage and necrosis of the hematopoietic tissue (and eventually the parenchyma) of the kidneys, liver, and spleen, with mononuclear infiltrates in later stages of the disease. Fragmentation of melanomacrophages in a wide range of tissues, particularly the liver, was a prominent feature in experimentally infected anurans. Interestingly, infection of splenic lymphocytes was only seen in frogs with hemorrhagic disease and not in those with ulcerative syndrome; hence, it was suggested that infection of the spleen is required for frogs to develop hemorrhagic disease. Lesions in salamanders range from foci of degeneration to larger areas of mucosal necrosis and ulceration and hemorrhage in the gastrointestinal tract. Focal or widespread necrosis occurs in the liver in hepatocytes and in associated hematopoietic tissue, intestines, kidneys, and spleen and other lymphoid tissues. Tadpoles show similar lesions but necrosis and an inflammatory response may be limited, particularly in animals dying peracutely.
Large basophilic to amphophilic intracytoplasmic inclusions are typically observed in necrotic areas of the kidney, liver, spleen, lymphoid, and hematopoietic tissues.1,16,21 In other cases, inclusions may not be seen, even though the virus is associated with the lesions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree