Chapter 25
Amphibian Therapy
Ranavirus
Ranavirus is a worldwide viral infection that may cause morbidity and mortality in most, if not all, species of amphibians and is covered elsewhere in detail in this text. It should be part of the differential diagnosis for any amphibian with erythema, petechia, ecchymoses, plaques, punctuate nodules, ulcers, exposed muscle or bones, hydrocoelom, lethargy, inappetence, tremors, seizures, opisthotonos, or sudden death (Figures 25-1, 25-2, and 25-3); in short, almost any ill amphibian should be suspected of having a ranavirus until proven otherwise. From a practical point of view, supportive care should begin while polymerase chain reaction (PCR) and other diagnostic tests are pending. Critical steps in managing a possible ranavirus-infected amphibian include providing a “hot spot” so that the amphibian may thermoregulate to reduce viral replication; a temperature of at least 26°C is needed for infected amphibians to have the highest chance of survival. Providing a localized warmer temperature (30°C to 40°C) may also improve survival chances. Another critical step is preventing fatal electrolyte imbalances with the use of a balanced electrolyte bath such as amphibian Ringer’s solution or, if that is not available, 0.6% saline solution (6 g of NaCl in 1 L distilled water). Amphibian Ringer’s solution is made by adding 6.6 g NaCl, 0.15 g KCl, 0.15 g CaCl2, and 0.2 g NaHCO3 to 1 L of distilled water. It may be stored for up to 7 days or longer if the solution can be autoclaved and stored in sterile containers. An alternative formulation consists of 6.5 g NaCl, 0.42 g KCl, and 0.25 g CaCl2 to 1 L of distilled water. Up to 10 g glucose may also be added to the solution, but this reduces the shelf life of unsterilized solution to 24 hours. Cutaneous respiration should be supported with an enriched oxygen environment, and secondary infection with bacteria and other pathogens should be prevented. Ulcers and open wounds can be managed with enrofloxacin/ silver sulfadiazine (Baytril Otic) and silver sulfadiazine cream. Compounded doxycycline topical gel has also been used for lesions because of its antibacterial and potential antiinflammatory properties. The goal is to apply 2.5 mg/kg to 5 mg/kg in total volume to the total number of wounds. In practice, many compounding pharmacies will only produce 1%, 2%, or 5% gels, so the dosing is often much larger than the goal. The wound should be patted dry before application, and the amphibian should be maintained on damp paper towels for at least 15 minutes after application of the gel. Provide nutrition via gavage with a highly digestible liquid diet (e.g., LaFeber’s Emeraid for Carnivores). The typical volume is 2% of the amphibian’s body weight and should be given every 5 days. Underlying malnutrition such a hypovitaminosis A should be addressed and inflammation reduced, but this step is controversial because human literature currently is in flux about the value of nonsteroidal antiinflammatory drugs. Further, not much is known about the COX-1 and COX-2 pathways in amphibians. This author’s impression is that meloxicam at 0.4 to 1 mg/kg applied intramuscularly or topically may help. He also administers doxycycline orally at 2.5 mg/kg per os (PO, by mouth) once daily and topically (see previously) for its antiinflammatory properties described in other species. Stocking density should be reduced. Most amphibian patients do best kept alone. Stress may be further reduced with minimal handling or observation other than what is essential for treatment.
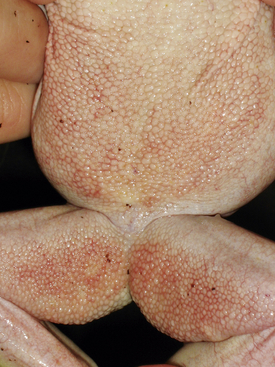
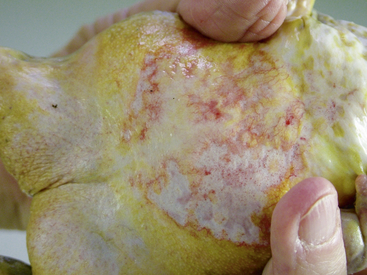
FIGURE 25-1 White’s Tree Frog (Litoria caerulea) with ventral erythema consistent with ranavirus infection.
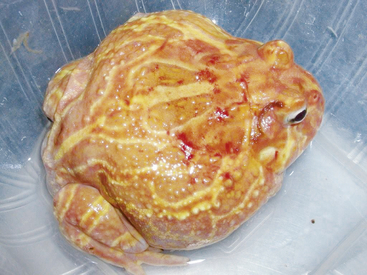
FIGURE 25-2 Albino Horned Frog (Ceratophrys ornata) with bleeding ulcers consistent with ranavirus infection.
There is some evidence that amphibians may acquire immunity to ranavirus (see Chapter 24, Ranaviruses), so culling of known ranavirus-positive amphibians may be counterproductive for managing a healthy captive population.
Grayfer, L., De Jesus Andino, F., Chen, G., et al. Immune evasion strategies of ranaviruses and innate immune responses to these emerging pathogens. Viruses. 2012; 4:1075–1092.
La Fauce, K., Ariel, E., Munns, S., et al. Influence of temperature and exposure time on the infectivity of Bohle iridovirus, a ranavirus. Aquaculture. 2012; 354-355:64–67.
Lesbarrères, D., Balseiro, A., Brunner, J., et al. Ranavirus: past, present and future. Biol Lett. 2012; 8:481–483.
Nazir, J., Spengler, M., Marschang, R. E. Environmental persistence of amphibian and reptilian ranaviruses. Dis Aquat Organ. 2012; 98:177–184.
Stöhr AC, Hoffmann A, Papp T, et al. Long-term study of an infection with ranaviruses in a group of edible frogs (Pelophylax kl. esculentus) and partial characterization of two viruses based on four genomic regions. Vet J, in press. Available online 25 March 2013, at http://www. sciencedirect. com/science/article/pii/S1090023313000762.
Chytridiomycosis
Batrachochytrium dendrobatidis is a major threat to the health of wild and captive amphibians worldwide. This fungus infects the keratinized epidermis of amphibians. Some amphibian species tolerate its presence, betraying little outward sign of infection other than increased frequency of shedding, while others show brown discoloration of the skin, erythema, excess keratinized skin buildup on the toe tips and ventral skin, and a hunched posture (Figures 25-4, 25-5, 25-6, and 25-7). Bufonids may develop erythema of the ventral drink patch and may soak in the water more than normal. Some chytrid-infected amphibians may die without outward signs of illness, which is apparently the result of a fatal electrolyte imbalance (low sodium, potassium, chloride, and magnesium). Known carrier species include African Clawed Frogs (Xenopus laevis), which are the presumptive origin of this disease, American Bullfrogs (Rana catesbeiana), and Tiger Salamanders (Ambystoma tigrinum), but it is likely that many other species are also carriers.

FIGURE 25-4 Hyperkeratosis of a White’s Tree Frog (Litoria caerulea) infected with chytrid. Note hunched posture.

FIGURE 25-7 Close-up view of excess skin shedding from two chytrid-infected White’s Tree Frogs from Figure 25-6.
Real-time PCR is the most sensitive antemortem diagnostic test for chytridiomycosis; other PCR techniques are used in many laboratories but are slightly less sensitive. Light microscopy evaluation of biopsy samples and wet mounts of skin scrapes miss all but very advanced infections.
Sugestions for Eliminating Chytrid from Amphibians
Itraconazole
Itraconazole is effective for juvenile and adult specimens of several species of amphibians, but causes death in larvae and recent metamorphs. Any hospital that treats amphibians should always have unexpired containers of 1% itraconazole (Sporonox) in its pharmacy. While compounded itraconazole has been widely used, there is some question as to its stability and activity between batches and between pharmacies. To make therapeutic 0.01% itraconazole solution, see Box 25-1.
Terbinafine
Terbinafine may be available as an over-the-counter medication to treat athlete’s foot in humans (e.g., Lamisil AT 1% spray). It may also be purchased through a compounding pharmacy (i.e., 10 mg/g terbinafine in ethanol). To make the therapeutic 0.01% solution of Terbenafine, see Box 25-2.
Chloramphenicol
Chloramphenicol is rarely used in the United States because of the risk of aplastic anemia developing in people who have contact with even miniscule amounts of this medication. Chloramphenicol appears to be safe and effective for all life stages of some amphibians and has a further advantage of treating some of the common secondary bacterial infections. The pure chloramphenicol powder may be purchased through a compounding pharmacy. Anyone handling the powder or therapeutic solution should wear disposable gloves. To make the therapeutic 30 ppm solution of chloramphenicol, see Box 25-3.