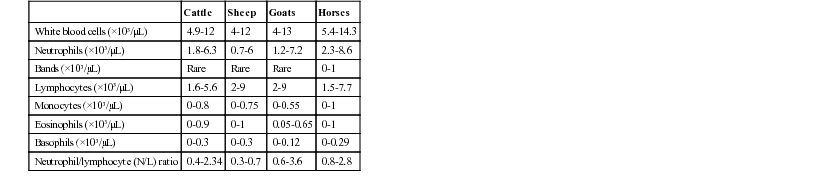
Leslie C. Sharkey, Jed A. Overmann *, Consulting Editors Interpretation of the leukogram in the context of the clinical history, physical examination, and the rest of the minimum laboratory database (complete blood cell count, serum biochemical data, and urinalysis) can provide valuable insights into the diagnostic process in large animal species. Often, leukogram data are used to evaluate patients for the presence of inflammation or hematopoietic neoplasia. Careful attention to sample collection and handling (see Chapter 23) will help ensure high-quality results. Key components of the leukogram include the total white blood cell count (WBC), differential cell counts including the absolute numbers of each leukocyte type, and a microscopic evaluation of the blood film. Blood film evaluation is necessary to visually verify the WBC, to generate the differential, and to evaluate the leukocytes for important morphologic abnormalities that contribute to the diagnostic interpretation of the quantitative data. Although the newer automated hematology analyzers will generate leukocyte differentials for numerous species, the Clinical Laboratory Standards Institute still considers the manual differential cell count to be the reference standard.1 Microscopic examination of the blood film for the presence of toxic change, band neutrophils, reactive changes in lymphocytes, neoplastic cells, and organisms is required for a full characterization of the leukogram. The concurrent measurement of acute phase proteins can augment the diagnostic interpretation of leukogram data. When evaluating the leukogram, clinicians should remember that each data set is a snapshot in time of a dynamic system in which the numbers of cells in the circulation reflect a balance between the release of cells from the bone marrow and egress of cells into the tissues. Serial monitoring of patients often provides more information than a single time point. It is also important to keep in mind that the numbers of cells trafficking in the circulation may not be reflective of the tissue content of cells; therefore, cytology or biopsy of tissues may be necessary to confirm the presence of inflammation or hematopoietic neoplasia. Leukocytes are partitioned into granulocytes (neutrophils, eosinophils, basophils) and mononuclear cells (lymphocytes, monocytes). In postnatal life, the main source of hematopoiesis is the bone marrow, except for lymphocytes, which migrate from the bone marrow to tissues such as the lymph nodes, spleen, and mucosal-associated lymphoid tissue after birth. The following sections will briefly describe the biology of each type of leukocyte, as well as the interpretation of high and low values and common morphologic abnormalities. Note that the reference intervals for some leukocyte subtypes encompass zero, making the detection of low values difficult and therefore the interpretation of questionable clinical significance (Table 25-1). Although reference intervals are provided here for guidance, clinicians should use laboratory-specific reference intervals because of the potential effect of instrumentation and geographic variation on leukocyte parameters. Other factors that influence reference intervals in large animal species include age, reproductive and lactation status, and other husbandry issues that are explored in further detail elsewhere.2–4 Neutrophils develop from hematopoietic stem cells in a process that usually takes about 6 days under the influence of an array of cytokines. Some species can accelerate the process in situations of increased demand, but that capacity appears to be limited in cattle.5 There is a storage pool of neutrophils in the marrow; however, once that is depleted, earlier precursors, including band neutrophils and sometimes metamyelocytes or even myelocytes, may be observed in the peripheral circulation in a phenomenon known as a “left shift.” Neutrophils circulate for only a few hours after release, existing in either the circulating pool where they can be measured, or in the marginal pool along the endothelial surface where they are not recovered during blood sample collection. Shifts between the circulating and marginal pools in response to hormonal mediators can result in rapid changes in neutrophil numbers measured in the leukogram. Neutrophils migrate into tissues, especially the gastrointestinal and respiratory tracts and the skin, through selectin-mediated rolling followed by integrin-mediated adhesion of neutrophils to the endothelium. Transmigration between or through endothelial cells allows neutrophils to enter the tissues, where they will bind to chemokines that direct them toward targets in the tissue. Receptor-mediated phagocytosis of bacteria and other particulate matter is followed by proteolytic digestion and/or the respiratory burst using granule contents. Neutrophils also amplify local inflammation and interact with cells of the adaptive immune response.6 These mechanisms can result in damage to normal tissues in addition to the killing of infectious agents, contributing to acute respiratory distress syndrome and multiple organ failure. Senescent neutrophils undergo apoptosis in tissues and are cleared by tissue macrophages. Some of the morphologic abnormalities that can be relevant to the interpretation of the leukogram in large animals include toxic change, Pelger-Huët and pseudo–Pelger-Huët anomalies, and the presence of intracellular organisms including Anaplasma phagocytophilum. The common components of toxic change include cytoplasmic basophilia and vacuolization and the presence of Dohle bodies in the cytoplasm of neutrophils; rarer manifestations include nuclear abnormalities, toxic granulation, and increased cell size. Toxic change is subjectively evaluated during the blood film evaluation and graded as 1+ to 4+. These changes occur in the neutrophil cytoplasm during the development in the marrow and signal the likelihood of inflammation; toxic change may be accompanied by a left shift. Pelger-Huët anomaly is an incidental asymptomatic congenital condition in which a defect occurs in the segmentation of leukocyte nuclei, giving the impression of a severe left shift in the absence of toxic change or other signs of inflammation.7 In contrast, pseudo–Pelger-Huët is pathologic hyposegmentation that occurs as a result of severe inflammation and is accompanied by toxic change and inflammatory disease. Anaplasma phagocytophilum is a tick-borne bacteria common to humans as well as small and large animal species that survives in neutrophils by avoiding the bactericidal mechanisms.8 Organisms are most likely to be identified microscopically in acute infection, when serologic methods for detection may still be negative. Eosinophil development in the marrow is very similar to that of neutrophils, although it occurs under the influence of distinctive cytokines. Normally, eosinophils spend a short time in the circulation before entering the tissues using similar mechanisms to neutrophils, although the numbers in the peripheral blood are usually quite small. The tissue survival time of eosinophils is usually short; however, they may persist in tissues for longer periods if appropriate survival factors are present. Recirculation is even possible under some conditions. Although eosinophils have the capacity for phagocytosis, more prominent functions include defense against parasites by externalization of granule contents and modulation of inflammation, particularly allergic and hypersensitivity disorders. Eosinophils are primarily tissue dwelling cells, so numbers in the circulation may not accurately reflect tissue eosinophilia. Basophil development largely parallels that of eosinophils, although it is associated with a specific growth factor repertoire. Basophils are the least numerous leukocyte in the peripheral blood under most conditions, where, like other granulocytes, they circulate briefly before entering tissues through similar mechanisms. With even less phagocytic and bactericidal potential than eosinophils, basophils are important in hypersensitivity responses and allergic conditions, which they mediate through release of their granule contents into the local environment. The accuracy of basophil counts by automated analyzers has been questioned. Lymphocytes encompass a heterogeneous group of mononuclear leukocytes fundamental to the adaptive immune response, although some, like natural killer cells, are aligned with innate immunity. Circulating lymphoid cells are generally divided into natural killer cells, B-cells, and T-cells, with an expanding number of T-cell varieties being characterized, each with a specific function. Advanced diagnostic techniques such as flow cytometry are required to identify cells from different lymphocyte subsets, which are not routinely distinguished during blood film evaluation. All originally derive from bone marrow stem cells; however, T-cells complete maturation in the thymus and participate in cell-mediated immunity, whereas B-cells develop in the marrow and other peripheral tissues to eventually disperse throughout the body to effect humoral immunity. Unlike most leukocytes in the peripheral blood, lymphocytes have significant potential for proliferation. Lymphocytes continually recirculate among tissues in a process called “trafficking,” which is most relevant for T-cells because B-cells release antibodies that are carried by the circulation to relevant sites. At any given time, only a tiny fraction of an animal’s lymphocytes are present in the circulation, so the peripheral blood lymphocyte count is not reflective of total body lymphoid activity. Likewise, advanced diagnostic techniques are required to characterize the lymphocyte subsets in circulation, which may change with different pathologic processes. Monocytes develop in the bone marrow and enter the circulation, where they persist longer than other leukocytes. Like other leukocytes, monocytes migrate into tissues; however, they have the potential to differentiate into a variety of macrophage subtypes (e.g., Kupffer cells, alveolar macrophages, microglial cells) depending on the local microenvironment. Monocytes can re-enter the circulation from the tissues. Unlike mature granulocytes that are post-mitotic, monocytes have a limited capacity for replication in tissues. The major functions of macrophages once in the tissues include inhibiting the growth of intracellular pathogens, antigen processing, removal of dead cells and debris, control of iron metabolism, and complex regulation of the inflammatory response. Depending on the stimuli, macrophages can develop into proinflammatory M1 macrophages or antiinflammatory M2 cells.9
Alterations in the Leukogram

Leukocytes
Neutrophils
Eosinophils
Basophils
Lymphocytes
Monocytes
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Alterations in the Leukogram
Chapter 25