Mats H.T. Troedsson, Bruce W. Christensen, Consulting Editors Stallions, bulls, rams, and bucks intended to be used as breeding animals need to have (1) normal genital organs, (2) the libido necessary to tease females and gain an erection, (3) the physical ability to mount and intromit the penis into the female’s vagina, and (4) an adequate number of morphologically normal, motile spermatozoa in each ejaculate to be considered as satisfactory breeders under natural service conditions. Digression from normal sexual function in males is usually recognized clinically by changes in sexual behavior, abnormalities or diseases of the genital organs, or a decreased pregnancy rate in dams bred. Subfertile males may be responsible for significant economic loss in the livestock industry. Sexual function may be altered by any of four major mechanisms: general physical abnormalities, abnormalities of the genital organs, decreased libido, and poor semen quality (Boxes 12-1 and 12-2). The male must be mobile enough, especially in a pasture breeding program, to locate, tease, mount, and breed estrual females successfully. Musculoskeletal abnormalities may limit reproductive ability or desire. Hindlimb conformation defects in bulls and rams, degenerative joint disease involving the hock in stallions, and foot problems in rams are examples of conditions that may cause enough discomfort to interfere with the normal breeding process or prevent normal mobility, impairing reproductive performance.1–3 Congenital or acquired abnormalities of the genital organs, including the penis, prepuce, scrotum, testicles, spermatic cords, or accessory sex glands, can lead to altered sexual function or infertility. Congenital abnormalities such as persistent penile frenulum and penile deviations in bulls may prevent normal intromission.4 Acquired lesions such as a penile hematoma caused by rupture of the tunica albuginea of bulls at time of service may limit sexual function by causing paraphimosis, adhesions, or sensory nerve damage.5 Libido is an essential component of breeding performance but may be difficult to measure during a breeding soundness examination and cannot be evaluated if semen is collected by electroejaculation. Libido has been demonstrated to be an inherited behavioral trait in bulls.6 Semen volume, concentration of spermatozoa, percentage of progressive motility, and percentage of morphologically normal spermatozoa are semen parameters commonly measured during a breeding soundness examination. Abnormalities of semen quality associated with decreased fertility in bulls include spermatozoa morphology and, to a lesser degree, motility.2,4,7 A complete breeding soundness examination and history should be obtained, including number of females bred each year, conception rates, breeding methods (natural service or artificial insemination), and results of previous breeding soundness examinations (Box 12-3). A medical or health history, including medications, vaccinations, and previous illnesses, should be obtained. The animal should be given a general physical examination. Hindlimb conformation and the presence of degenerative joint disease, laminitis, foot abscesses, abnormal foot wear, corkscrew claw defect, weak pasterns, postleggedness, sickle hock, interdigital fibromas (bulls), foot rot, ulcerative dermatitis and pizzle rot (rams), and caprine arthritis-encephalitis (CAE) (bucks) should be noted. An ophthalmologic examination should be done to ensure that the animal has adequate vision and that no significant pathologic condition is present. A special emphasis is placed on identification of squamous cell carcinoma and pinkeye (Moraxella bovis) and corneal lesions in bulls. Range animals showing weight loss and a decline in reproductive performance should be given an oral examination, and the parasite control program should be evaluated. The external genital organs should be examined carefully. The penis of the stallion is easiest to examine after an erection is obtained by teasing to an estrual female. In ruminants manual palpation of the penis per rectum or by use of an electroejaculator is suitable in many cases. The penis should be normal in size and shape and free of lesions. In bulls, deviations or other abnormal configurations such as corkscrew penis may occur with use of an electroejaculator and therefore cannot be considered abnormal.5 Rams and bucks should be carefully examined for abnormalities of the urethral process, including the presence of calculi.8 The lesions most often observed on the penises of stallions are squamous cell carcinoma and cutaneous habronemiasis.9,10 The prepuce should also be examined for lesions. Strictures of the preputial orifice may increase the risk of phimosis or paraphimosis. Bulls of the Bos indicus breeds often have a pendulous prepuce that is predisposed to traumatic injury, abscessation, stricture formation, and eversion.5,11 Ulcerative posthitis (pizzle rot) caused by Corynebacterium renale in rams on a high-protein diet is the most common lesion of the prepuce in rams.12 The scrotum, testicles, and spermatic cords should be examined for size, consistency, symmetry, and presence of lesions. Two scrotal testicles should be present, each smooth, resilient on palpation, and freely movable. Testes volume and consequently the amount of testicular parenchyma present are highly correlated with daily sperm production in all species. Each gram of testicular tissue should produce 15 to 20 million sperm per day. In ruminants, scrotal circumference has been determined to be highly correlated with testes weight or volume. Yearling beef bulls should have a scrotal circumference of 30 cm or more, depending on the animal’s age and breed.7 In stallions, measurements of total scrotal width or, more accurately, testicular volume have been evaluated and correlated to potential daily sperm production. Mature stallions should have a scrotal width of at least 8 cm. Testicular volume in stallions can be determined by the following steps13: 1. Measure the length (L), width (W), and height (H) of each testicle. 2. Volume of each testicle can be determined using the formula: 3. Add the volume of each testicle to obtain total testicular volume. The epididymides should be palpated for position, size, and presence of lesions. The most common palpable abnormality in rams is epididymitis caused by infection with Brucella ovis, Actinobacillus seminis, or Histophilus ovis.8,12,14 A definitive diagnosis is obtained by isolation of bacterial organisms in the semen and serologic testing. Diseases of the accessory sex glands are diagnosed most frequently in bulls. Vesicular gland adenitis is clinically recognized by the presence of leukocytes in the semen and enlargement, induration, and loss of lobulation noted during palpation and ultrasonographic examination of the glands per rectum.15,16 Libido and the ability to mate should be assessed after the physical examination. The male should be teased to a female in estrus. Interest in and interactions with the female and ability to gain an erection, mount, intromit the penis into the vagina (or into an artificial vagina), and ejaculate are noted. Libido and mating ability cannot be evaluated when using an electroejaculator to collect semen from ruminants. Tests for “serving capacity” have been described for bulls. The evaluation of semen quality is a major part of the breeding soundness examination. Semen from stallions is collected into an artificial vagina. Semen from ruminants may be collected into an artificial vagina or obtained by use of an electroejaculator. Semen collected by electroejaculation usually has a higher volume and a lower concentration of spermatozoa than semen collected by an artificial vagina. Semen quality in ruminants is scored primarily on the basis of motility and morphology.7 Evaluation of semen from stallions should include determination of volume, concentration, motility, and morphology. The motility of the spermatozoa should be evaluated microscopically on raw and extended semen immediately after collection. In ruminants, bright-field microscopy at ×40 to ×125 magnification is used to detect mass motion or swirling to evaluate gross motility. Changes in sperm concentration, progressive motility, or speed of progression of spermatozoa will decrease or eliminate the swirling effect. Phase contrast microscopy at ×200 to ×500 is used to evaluate motility of individual spermatozoa. Computer-assisted semen analysis (CASA) is commonly used at larger reproduction facilities. Concentration can be measured by use of a hemocytometer or a calibrated spectrophotometer. Morphology can be evaluated microscopically, using stained semen samples (e.g., eosin-nigrosin stain) or phase-contrast microscopy. Ejaculates from stallions may be collected once daily for 5 to 10 days until daily sperm output (DSO) is achieved to fully evaluate potential fertility.17 However, this is time-consuming, labor-intensive, and expensive. Consequently, most stallions are evaluated by the collection of two ejaculates 1 hour apart, with the total number of progressively motile, morphologically normal spermatozoa in the second ejaculate most critically evaluated. The age of the stallion or male ruminant being evaluated for potential fertility may influence the semen parameters, measurements of the testicular size, mating ability, and libido. Puberty is attained in the stallion at 18 months, in the bull at 9 to 12 months, and in the ram and buck at 7 to 8 months.18 Semen parameters and testicular size continue to increase until sexual maturity is reached. The season of the year in which the fertility evaluation is done may affect semen parameters in the stallion, ram, and buck. Microbiological samples should be routinely collected when evaluating infertility in the stallion and when evaluating high-risk populations of bulls. Smegma samples should be collected from the prepuce of bulls and cultured for Trichomonas foetus and Campylobacter fetus. Swabs from the preejaculate and postejaculate urethra, semen, fossa glandis, and prepuce of stallions should be cultured for potentially pathogenic bacterial organisms, especially Taylorella equigenitalis, Pseudomonas aeruginosa, and Klebsiella pneumoniae.9 The semen of rams should be cultured for B. ovis, A. seminis, and H. ovis.8,12,14 Serologic testing for exposure to equine arteritis virus (EAV), the causative agent of equine viral arteritis (EVA), is important for breeding stallions. Determination of negative serologic status should be required before vaccination against the virus may be performed. Other tests that are occasionally performed to evaluate male reproductive function, health, or pathology include hormone analysis, chemical evaluation of seminal plasma, transmission electron microscopy of semen, karyotype, sperm chromatin structure assay, urethral endoscopy (stallion), and testicular biopsy. After summarizing the results of the entire breeding soundness evaluation, stallions and male ruminants may be categorized into classifications such as satisfactory, questionable, or unsatisfactory. In bulls a fourth category of “decision deferred” may be used for young prepubertal bulls or mature bulls that have recently experienced a transient disturbance in spermatogenesis. It must be emphasized that the breeding soundness examination is a measure of potential fertility.19 True fertility can be determined only by the results of breeding trials or by conception and live birthrates in dams bred. Claudia Klein Cyclic irregularities become evident in the display of irregular estrous cycles (i.e., an abnormal interval from one estrus to the subsequent estrus). Irregular estrous cycles can be physiologic such as during the transition period in seasonal breeders, can be pathologic such as a sequel to endometritis, or may be an artifact such as failure in estrus detection (Box 12-4).
Alterations in Sexual Function

Alterations in Male Sexual Function
Mechanisms of Altered Male Sexual Function
Approach to Diagnosis of Altered Male Sexual Function
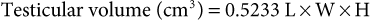
Cyclic Irregularity



