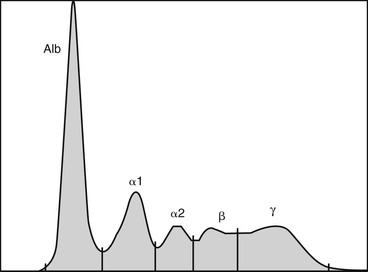
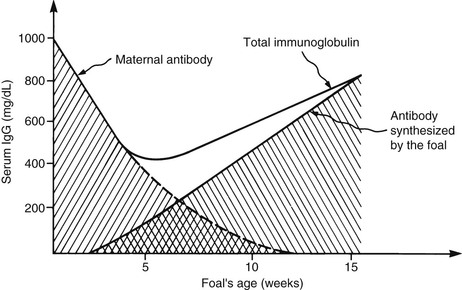
Jennifer L. Johns *, Consulting Editor Serum or plasma protein measurements constitute a vital component of laboratory diagnostic evaluations. Globulin and total protein measurements will be higher in plasma than serum because of the presence of coagulation proteins, particularly fibrinogen. It is vital to use appropriate reference intervals for the sample type. The following measurement techniques are widely used (units for the following serum or protein measurements are g/dL or the SI unit g/L): Refractometry: The total protein concentration of serum, plasma, or body fluids can be determined via refractometry. The principle of measurement is that the degree of light refraction by a solution is proportionate to the concentration of solids. Refractometers are calibrated under the assumption that the solids are entirely proteins. However, other substances can increase the refractive index of a solution and falsely increase the total protein reading (e.g., marked hyperglycemia with glucose concentration of 700 mg/dL or azotemia with blood urea nitrogen concentration of 300 mg/dL can falsely increase the protein reading by 0.6 g/dL).1 Hemolysis does not alter refractometer protein readings. The effect of marked icterus is unclear.2 Biuret reaction: Serum protein is commonly measured via modified biuret testing on automated analyzers. The principle involves basic spectrophotometry measuring color change following copper binding to peptide bonds. Depending on the specific assay, hemolysis can cause a positive interference (e.g., hemoglobin of 400 mg/dL can cause a 12% positive bias).3 This method is not sensitive to very low protein concentrations (e.g., in cerebrospinal fluid). BCG albumin binding: Albumin concentration in serum or plasma is usually measured via the bromocresol green (BCG) reaction. The principle involves basic spectrophotometry measuring color change when BCG binds to albumin. Nonspecific BCG binding to globulins can artifactually inflate the albumin value, particularly with a very low albumin concentration.4 Plasma albumin values can be increased over serum values because of nonspecific plasma globulin binding. Also, heparin interference can increase or decrease an albumin reading depending on the assay.5 Globulins are typically calculated by subtracting the albumin value from the total protein value. Protein electrophoresis (PE): Serum or plasma proteins can be separated into albumin and globulin fractions electrophoretically. The principle of measurement is that an electrical charge is applied to a cellulose acetate or agarose gel matrix, allowing serum/plasma proteins to migrate through the matrix and separate into bands based on their charge and size. The acetate or gel is stained to detect the protein bands and read via densitometry to generate a tracing. Albumin has a low molecular weight (roughly 66 kD), is highly anionic, and migrates the farthest to form a band near the anode. The identical nature of all albumin molecules and therefore their migration to a single band generates a single sharp, narrow-based peak on the tracing. Globulins are divided into α-, β-, and γ-globulin regions in ruminants. In horses, globulins are further divided into α1-, α2-, β1-, β2-, and γ-globulin regions (Fig. 26-1). The heterogeneity of globulin fractions results in broad-based peaks for each, as the diverse proteins in each region migrate slightly differently. Rarely, a narrow-based peak will be seen in the γ-globulin region (see Hyperglobulinemia section). The presence of coagulation proteins will cause higher globulin peaks on protein electrophoresis of plasma (PPE) versus serum (SPE). In particular, fibrinogen will migrate into either the “late-beta” peak or the β-γ junction, and in rare cases might obscure the presence of a monoclonal peak.6,7 Both α- and β-globulins include many acute phase proteins (see later), whereas immunoglobulins (antibodies), primarily IgG, comprise the γ-globulins. IgA and IgM are immunoglobulins that migrate in the β2 or β-γ junction regions, but they are not detected in health via PE testing. Immunoelectrophoresis: Immunoelectrophoresis is a specialized technique for quantification of each immunoglobulin subtype (e.g., IgG, IgM) via electrophoresis followed by immunoprecipitation using subtype-specific antibodies. Units are usually given in mg/dL. Other immunoglobulin measurements are designed specifically for detection of IgG in neonatal samples and are discussed under failure of passive transfer (see Chapters 19 and 53). Reference values for proteins vary by species (Table 26-1). When evaluating protein concentrations in a patient, some clinicians use the ratio of albumin to globulins (A : G ratio). However, it is often simpler to evaluate albumin and globulins separately because the A : G ratio can change with alterations in one or both parameters. Biologic variation with life stage: Transfer of immunoglobulins from the dam to the neonate via colostral ingestion is a key process that determines the neonate’s subsequent immunocompetence and is often reflected in serum globulin levels in the first few weeks of life. In addition, neonates generally have lower serum albumin levels; for example, calves had decreased albumin values in the first 8 days of life.8 Total protein levels in calves are decreased at birth, rise immediately following colostrum intake, then fall below the reference interval again at about 2 weeks of age and remain subnormal for some time.8 IgG measurement also reflects the combined presence of maternal and neonatal proteins (Fig. 26-2). Studies in cows found gradually increasing plasma globulin levels during pregnancy until 4 weeks pre-parturition, then a steady drop until parturition.9 IgG1 production is greatly increased at parturition and the half-life is decreased, corresponding with transfer to colostrum.10 Serum total protein steadily increased during lactation in Danish landrace goats.11 In pregnant and lactating mares, significant differences in albumin, globulins, and total protein were not found at any stage.12 Aging can be associated with alterations in some laboratory parameters. However, no significant differences in biochemical parameters or specific concentrations of IgG, IgA, or IgM were found in aged horses, despite alterations in lymphocyte populations.13,14 Hyperproteinemia can result from an increase in both albumin and globulins (panhyperproteinemia) or only globulins (hyperglobulinemia) (Boxes 26-1 and 26-2). An increase in the concentration of all blood proteins results from hemoconcentration. Dehydration (resulting from decreased fluid intake, excessive fluid loss, or both) causes panhyperproteinemia with an associated increase in packed cell volume (PCV). This is due to a relative increase in protein and RBC concentrations as they become more concentrated in blood. True hyperalbuminemia (i.e., increased albumin synthesis) does not occur. Hyperalbuminemia is an insensitive indicator of dehydration. If the true albumin level is decreased, a dehydrated patient may have a normal albumin result, masking effects of hemoconcentration. Similarly, a dehydrated, anemic animal may have hyperproteinemia with a normal or decreased PCV. In an animal with a plasma protein concentration of 7.0 g/dL in health, a 10% decrease in plasma volume (severe dehydration) will result in a plasma protein concentration of 7.8 g/dL.2 As with albumin measurement, the total plasma protein measurement will underestimate the degree of dehydration if the patient is losing protein along with fluid. This is relatively common in renal disease, in protein-losing enteropathy with diarrhea, and in third-space loss when fluid and protein accumulate in the thoracic and/or abdominal cavity. Dehydration alone can result from GI fluid sequestration, including intestinal obstruction, vagal indigestion with internal vomiting, and grain engorgement. Less common causes of dehydration include polyuria with renal failure, exudation from extensive skin wounds, and excessive sweating. Physiologic responses to dehydration include increased urine concentration and decreased urine output, increased fluid absorption from the gastrointestinal (GI) tract, and increased thirst. The most common cause of hyperglobulinemia with normal hydration status (i.e., without hemoconcentration) is inflammation resulting in increased production of numerous globulins, including many acute phase proteins (see Acute Phase Response section). Chronic inflammation frequently results in a nonspecific increase in γ-globulins (polyclonal gammopathy) resulting from production of different immunoglobulins by plasma cells in response to chronic antigenic stimulation. A broad-based peak will be seen on SPE in the γ region, and can extend into the β region as a result of production of IgM and/or acute phase β-globulins. An increase in both β- and γ-globulin fractions (β-bridging) can occur with intense antigenic stimulation, chronic active hepatitis, or lymphoma. Increased IgG in the γ-globulin fraction is termed hypergammaglobulinemia and can be seen in a range of chronic inflammatory diseases, including internal abscessation, chronic hepatitis, immune-mediated diseases (e.g., immune-mediated hemolytic anemia or thrombocytopenia), and lymphoma. Infection with the agent of epizootic bovine abortion triggered significant increases in IgG in clinically affected heifers.15 Increased production of a single immunoglobulin by a clonal population of B lymphocytes/plasma cells is known as a monoclonal gammopathy. On SPE, the monoclonal peak is as narrow-based and sharp as the albumin peak as a result of the identical migration of the monoclonal immunoglobulins (also known as paraproteins). Monoclonal gammopathies are usually the result of neoplasia of B lymphocytes or plasma cells, including B-cell lymphoma, multiple myeloma, and B-cell lymphocytic leukemia. Increased susceptibility to hemorrhage can occur with monoclonal gammopathy as a result of impaired platelet function caused by immunoglobulin coating, and it can be exacerbated by hyperviscosity syndrome. Acute inflammatory processes can trigger an acute phase response. Infectious causes are common but many noninfectious causes (e.g., trauma, strenuous exercise) are also documented. The body’s response to the inflammatory insult is altered liver production of many proteins, termed acute phase proteins (APPs). A few proteins are negative APPs and their production decreases with acute inflammation. Albumin is the most prominent negative APP, and levels will decrease in circulation proportionate to its half-life in each species. For example, the albumin half-life is 19.4 days in horses and 16.5 days in cattle, so hypoalbuminemia caused by inflammation will take several weeks to develop in these species.4,16 Transferrin and transthyretin (prealbumin) are other negative APPs, as is apolipoprotein A-1 (see following sections on specific APPs). The presumed role of decreased production of certain proteins is to shift available amino acids to the synthesis of proteins that are useful in host defense.17 Most reactants are positive APPs, meaning production increases with inflammation. They migrate in the α- and β-regions on SPE or PPE. The positive APPs are a diverse group including proteins involved in coagulation, opsonization, iron regulation, and limiting of tissue injury. Protein electrophoresis can reveal increased globulin fractions that are comprised largely of APPs. Some diseases cause fairly specific alterations (e.g., internal parasitism in diarrheic horses caused increased β-1 globulin levels that were significantly higher than those in diarrheic horses with other disorders).18 Nonprotein assays are also used to diagnose an acute phase response (e.g., serum iron outperformed plasma fibrinogen as an indicator of acute inflammation in horses).19 Measurement of individual APPs can be useful diagnostically and is not limited to blood; assays for APPs in milk and saliva have also proven useful.20–22 The following is a list of major APPs in large animals. Fibrinogen is a useful marker of inflammatory disease in large animals. Increased plasma fibrinogen, termed hyperfibrinogenemia, can occur in a myriad of conditions. Fibrinogen is semiquantitatively measured via the heat precipitation method, along with total plasma protein, as part of the routine CBC. Serum samples cannot be used to measure fibrinogen. The heat precipitation method cannot be used to detect hypofibrinogenemia (see Chapter 27). As for other proteins, fibrinogen can be increased as a result of dehydration. Calculating the plasma protein : fibrinogen (PP : F) ratio is useful when fibrinogen is increased. If the PP : F is greater than 15, hyperfibrinogenemia is due to dehydration. If the PP : F is less than 10, the patient has true hyperfibrinogenemia (caused by inflammation).23 Severe inflammatory disease in horses and ruminants consistently causes hyperfibrinogenemia, although the increase in fibrinogen may not be proportionate to the degree of inflammation. Increased fibrinogen often precedes development of an inflammatory leukogram in ruminants, particularly in cattle.23 Hyperfibrinogenemia can persist for a period of time postoperatively. In one study, more than half of horses undergoing subchondral bone cyst removal still had hyperfibrinogenemia 15 days postoperation24; in another, fibrinogen remained increased through day 11 following laparotomy and other procedures.25 In adult horses, a number of conditions including colic, sterile inflammation, surgical intervention, and chronic internal abscessation are all associated with hyperfibrinogenemia (Box 26-3).24,26–28 Vaccination of horses induces an acute phase response including increases in both fibrinogen and serum amyloid A.29 Fibrinogen response in foals is more variable. In healthy neonatal foals, fibrinogen levels are initially lower than adult values and then equilibrate within a few weeks of age.30,31 Hyperfibrinogenemia lags behind leukocytosis as an indicator of inflammation.30,32 Fibrinogen was an unreliable marker of foal sepsis in one study,33 although marked hyperfibrinogenemia of 900 to 1500 mg/dL had utility as a diagnostic marker of foal osteomyelitis.34 Fibrinogen may be useful as an indicator of foal pneumonia resulting from Rhodococcus equi; one study found that hyperfibrinogenemia had greater specificity than leukocytosis, but another reported better diagnostic performance (encompassing both sensitivity and specificity) for leukocytosis over hyperfibrinogenemia.35,36
Alterations in Blood Proteins

Hyperproteinemia
Panhyperproteinemia
Hyperglobulinemia
Acute Phase Response
Fibrinogen