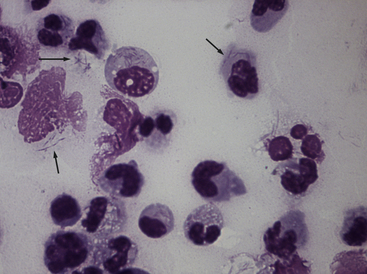
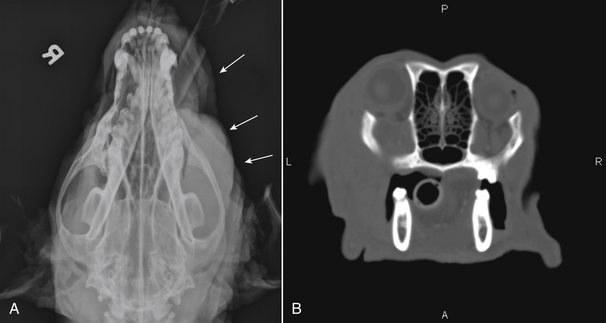
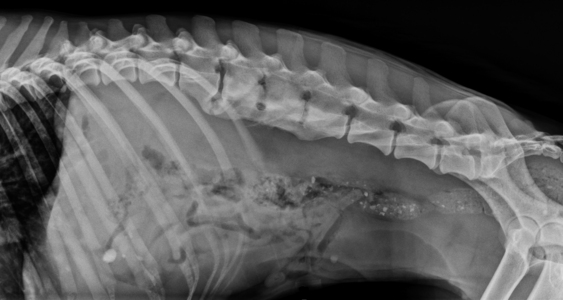
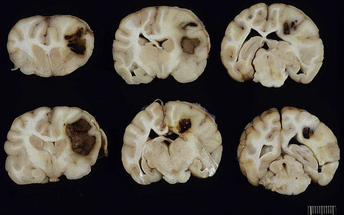
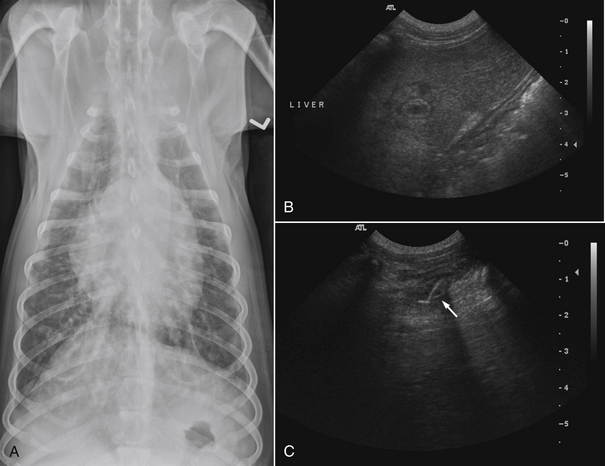
Chapter 42 Actinomycosis is caused by anaerobic or microaerophilic, filamentous, gram-positive bacteria that belong to the genus Actinomyces (and to a lesser extent, the related genus Arcanobacterium). These organisms are normal inhabitants of mucous membranes, especially of the oropharynx, but also the genital and gastrointestinal tracts, and cause opportunistic infections. Together with other oral commensal bacteria, actinomycetes colonize the periodontal mucosal surfaces and adhere to the tooth surface to form plaque. Numerous Actinomyces species have been cultured from the mucous membranes, dental plaque, and saliva of healthy dogs and cats. Organisms isolated from ill dogs and cats with actinomycosis are shown in Box 42-1.2–15 Usually, actinomycosis develops when Actinomyces spp. are inoculated into tissues along with other bacteria, often as a result of a deeply penetrating wound or foreign body migration. Young adult to middle-age large-breed dogs that have outdoor access are often affected, especially retriever and hunting breeds.16,17 There is no clear sex predisposition, and the median age of dogs with actinomycosis is approximately 5 years.18 Actinomycosis in outdoor dogs is often related to exposure to penetrating plant foreign bodies such as grass awns. In cats, actinomycosis usually follows bite wounds and so is more commonly diagnosed in males.18,19 In the author’s hospital, two-thirds (11 of 17) cats with actinomycosis are male. However, underlying retrovirus infection is uncommonly present and does not appear to predispose to disease. After ingestion or inhalation, plant awns become contaminated with Actinomyces spp. and other bacteria from the oropharynx, and then migrate to various sites and act as a nidus of infection. Alternatively, organisms can be introduced into tissues at the time of a bite wound injury. The latter is the most common route of infection in cats, and can manifest as pyothorax, peritonitis, or cellulitis.18,19 Other co-infecting aerobic and anaerobic bacteria from the oral cavity or intestinal tract (“companion microbes”) undermine normal host defenses and reduce oxygen tension, which allows Actinomyces spp. to persist. Actinomyces spp. that possess fimbriae can bind to specific cell surface receptors on other bacteria, especially streptococci. This bacterial co-aggregation inhibits the ability of neutrophils to phagocytize the organisms.20 Dense colonies of Actinomyces spp. form, and these, together with other associated bacteria, are surrounded by neutrophils, macrophages, and plasma cells, with slowly progressive pyogranulomatous inflammation (Figure 42-1). Large aggregations of organisms form “sulfur granules,” which are tan to yellow colonies of actinomycetes, which can be microscopic or visible grossly. Proteolytic enzymes from the associated bacteria, macrophages, and degranulated neutrophils destroy connective tissue, which facilitates extension of the disease through normal tissue planes. Less often, organisms spread hematogenously to distant sites. In some cases, the inflammatory reaction is accompanied by mass formation and extensive fibrosis. The center of the lesions can eventually suppurate and soften, or draining tracts may develop. The tracts can close and reappear over weeks to months as infection gradually spreads through tissues. FIGURE 42-1 Abdominal fluid cytology from a 3-year-old male neutered domestic longhair cat with a 1-week history of progressive lethargy and anorexia, ascites, hyperbilirubinemia, and hypoproteinemia. Abdominal fluid analysis revealed a total protein of 2.2 g/dL, and 75,500 nucleated cells/µL, with 92% neutrophils and 8% large mononuclear cells. High numbers of moderately degenerative neutrophils were present with low numbers of macrophages. Moderate numbers of filamentous beaded bacteria were present both in the background and within phagocytes (arrows). Actinomyces spp. and Fusobacterium nucleatum were isolated from the peritoneal fluid. Necropsy revealed severe, chronic peritonitis with intralesional filamentous bacteria. The most common clinical forms of actinomycosis in cats and dogs involve the cervicofacial region, thorax, abdomen, and subcutaneous tissue, but central nervous system (CNS) infections (meningitis and meningoencephalitis) and ocular infections (keratitis and endophthalmitis) can also occur.21,22 Cervicofacial actinomycosis can follow bite wounds, perforation of the oropharynx by a foreign body, or chronic periodontal disease. The mandibular, submandibular, and ventral or lateral cervical areas are most frequently affected, but infections that involve the face, retrobulbar space, and temporal area also occur (Figure 42-2, A and B). Cutaneous-subcutaneous actinomycosis typically involves the lateral thoracic wall, flank region, and occasionally the limbs. Lesions on the thoracic or abdominal walls may represent extensions of thoracic, abdominal, or retroperitoneal actinomycosis. FIGURE 42-2 Cervicofacial actinomycosis in an 8-year-old female spayed Labrador retriever with slowly progressive, left-sided facial swelling. Skull radiograph (A) and computed tomographic scan (B). There was severe, regional soft tissue swelling over the left side of the face, which involved the left masseter muscle and zygomatic salivary glands and extended into the retrobulbar space, with secondary exophthalmos. Multiple premolars and molars of the left maxillary arcade were missing. Biopsy of the lesion revealed severe, diffuse, pyogranulomatous cellulitis with intralesional gram-positive, acid-fast negative bacilli. Culture yielded Actinomyces hordeovulneris (based on 16S rRNA gene sequencing), Pasteurella multocida subsp. multocida, Neisseria canis (based on 16S rRNA gene sequencing) and an Eikenella-like organism. Thoracic actinomycosis may be limited to the lung parenchyma but can involve other thoracic structures, such as the mediastinum, pleura, pericardium, and thoracic wall. It usually follows aspiration of oropharyngeal material, often together with a contaminated grass awn. Alternative routes of thoracic infection include mediastinal involvement after esophageal perforation or direct extension of subcutaneous or abdominal disease. Clinical signs include cough and less commonly hemoptysis, tachypnea, respiratory distress, and subcutaneous soft tissue masses or draining skin lesions on the thoracic wall. Sometimes there is a history of neck pain, gagging, or hypersalivation before the onset of tachypnea, possibly due to passage of a penetrating foreign body. Abdominal actinomycosis develops when ingested foreign bodies penetrate the gastrointestinal tract, which leads to the formation of intra-abdominal mass lesions and ascites.2,16,23 Abdominal involvement may also follow direct extension from subcutaneous tissues or hematogenous spread of the organism to abdominal organs such as the liver. Although dogs are more often affected than cats, intra-abdominal actinomycotic masses can occur in cats.24,25 Retroperitoneal actinomycosis in dogs often follows the migration of plant awns through the lung and up the crus of the diaphragm to its dorsal attachment. Initially, fever of unknown origin may be the only clinical sign. Ultimately, progression can lead to osteomyelitis and compression fractures of the vertebral bodies, with spinal pain and paresis or paralysis of the pelvic limbs (Figure 42-3).3,26,27 FIGURE 42-3 Left lateral spinal radiograph from a 3-year-old female Border collie with retroperitoneal actinomycosis. The dog was seen for a 3-day history of lethargy, fever, abdominal pain, and progressive pelvic limb paresis. Severe, smoothly marginated ventral spondylosis bridges the L1-L4 space. There is also marked irregular osteolysis of the 2nd, 3rd, and 4th lumbar vertebrae. There is loss of visualization, narrowing, and irregularity of the intervertebral disc spaces at L2-3 and L3-4. There is marked loss of detail within the retroperitoneal space, and the colon is displaced slightly ventrally. Irregular mineral foreign bodies are present in the stomach and colon. Although rare, actinomycosis of the brain and meninges can also occur in dogs (Figure 42-4).28–30 Brain abscesses may follow hematogenous dissemination from a distant site, or meningitis may result from extension of infection from an adjacent site, such as the middle ear.29,31 In humans, risk factors for CNS actinomycosis include dental disease or tooth extraction, head trauma, gastrointestinal tract surgery, and chronic otitis, mastoiditis, or sinusitis. CNS actinomycosis has been reported in dogs in association with concurrent otitis media/interna30 and head trauma,28 and in cats in association with retrobulbar abscesses31 and cutaneous abscesses of the tail base.13,32 FIGURE 42-4 Necropsy findings in a 1-year-old male neutered Yorkshire terrier with suspected CNS actinomycosis. Three weeks previously, surgery to correct an extrahepatic portosystemic shunt and remove a urate cystolith had been performed. The dog’s appetite and activity level did not improve after the surgery, and for the week before the dog was reexamined, disorientation and weakness was noted. The dog deteriorated rapidly after it was hospitalized, and euthanasia was performed. Three separate abscesses were found in the left cerebral cortex. Histopathology revealed neutrophilic inflammation, hemorrhage, and granular mats of material that contained gram-positive, acid-fast negative branching bacteria (consistent with Actinomyces spp.), as well as a dense population of gram-negative, Giemsa-positive cocci. (Courtesy University of California, Davis, Veterinary Anatomic Pathology Service.) Rarely, Actinomyces infections of the urinary bladder,12,33 gallbladder,34,35 and heart valve4,10,36,37 have been recognized in dogs, cats, and human patients. Lesions in dogs and cats with cervicofacial or cutaneous-subcutaneous actinomycosis may fluctuant or firm, indurated, have draining sinuses, and rarely are ulcerated. Pain and fever are variable. Thoracic or abdominal actinomycosis is often accompanied by a thin body condition and fever. Dogs and cats with thoracic actinomycosis may show tachypnea or respiratory distress, and thoracic auscultation may reveal decreased lung sounds if pyothorax or intrathoracic mass lesions are present. Masses and/or ascites may be detected on abdominal palpation of dogs with abdominal actinomycosis. Dogs with retroperitoneal actinomycosis can show pain on palpation of the abdomen or spine; signs of pelvic limb paralysis or paresis may be also present, such as pelvic limb ataxia, delayed or absent placing reactions, increased segmental reflexes, and loss of pain sensation. Neurologic signs in dogs with Actinomyces meningitis or encephalitis include altered behavior, decreased consciousness, cervical pain, vision loss, ataxia, tetraparesis, and seizures. In the human literature, actinomycosis has been referred to as the “most misdiagnosed disease.”38 Fibrous masses are frequently mistaken for neoplastic lesions, although reports of Actinomyces spp. infections that complicate neoplasia exist in human medicine.39,40 Aspiration of firm lesions is often unrewarding, organisms are often difficult to isolate from lesions, and short periods of antimicrobial drug treatment are ineffective, which all serve to further mislead the clinician to a diagnosis of neoplasia. Actinomycosis should be considered on the list of differential diagnosis in any animal with a history of penetrating plant awn foreign bodies, draining skin lesions, chronic fibrous masses, persistent bite wounds, pyothorax, retroperitoneal abscesses, or focal neurologic signs, especially when young-adult to middle-aged dogs are affected. Ultimately, diagnosis is based on clinical signs, identification of organisms with typical morphology with cytology or histopathology, and culture. Cytologic examination of aspirates of abscesses, effusions, or CSF from animals with actinomycosis typically reveals a suppurative to pyogranulomatous inflammatory response (>75% neutrophils). Total protein in pleural fluid is generally greater than 3.0 g/dL, with erythrocyte and nucleated cell counts often greater than 70,000 cells/µL. CSF from dogs and cats with cerebral actinomycosis can grossly resemble pus. Aspirates of firm masses may yield only a small amount of blood. Sulfur granules may be visible in effusion fluid or purulent material from cutaneous lesions, grossly as white to tan to gray granules, or microscopically as dense clusters of organisms. When bacteria are visualized, they may be filamentous rods suggestive of Actinomyces spp., or “companion” microorganisms. Actinomycetes are gram-positive, non-acid-fast filamentous organisms that are occasionally branched. The filaments are less than 1 µm wide, vary in length, and can stain irregularly, producing a beaded appearance (see Figure 42-1). Nocardia spp., Corynebacterium spp., and Mycobacterium spp. can be confused with Actinomyces species. Radiographs of cervicofacial or cutaneous-subcutaneous lesions may show evidence of adjacent osteomyelitis and/or periosteal new bone formation. Pulmonary involvement may manifest as interstitial or alveolar infiltrates, sometimes with consolidation (Figure 42-5, A). Air bronchograms may be seen within pulmonary mass lesions, which suggests the presence of a nonneoplastic process. Other variable findings include pleural thickening, pleural effusion, widening of the mediastinum, pleural mass lesions, enlargement of the cardiac silhouette (with pericardial involvement), and periosteal new bone formation or osteomyelitis involving adjacent ribs, vertebral bodies, or sternebrae. Dogs with retroperitoneal actinomycosis may have periosteal new bone formation on the ventral aspects of two or three adjacent vertebral bodies (usually T13 through L4) on abdominal radiography; involvement of disc spaces is uncommon (see Figure 42-3). Abdominal actinomycosis may be characterized by loss of abdominal detail due to peritoneal effusion and intra-abdominal mass lesions. FIGURE 42-5 Imaging findings in a 1-year-old female spayed Labrador retriever with systemic actinomycosis secondary to grass awn migration. A, Dorsoventral thoracic radiograph. Patchy diffuse interstitial opacities were present in all lung fields. B, Ultrasound image of the liver. Within the left liver, there were focal, ill-defined heteroechoic or hypoechoic nodules. One of these areas (shown) had a hyperechoic rim with echogenic luminal contents. The nodules were consistent with multifocal hepatic abscesses. C, Ultrasound image of the thoracic wall. A linear plant awn foreign body was identified (arrow).
Actinomycosis
Etiology and Epidemiology
Clinical Features
Physical Examination Findings
Diagnosis
Laboratory Abnormalities
Cytologic Examination of Body Fluids
Diagnostic Imaging
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Actinomycosis
Only gold members can continue reading. Log In or Register to continue
