Chapter 59 Acid-Base Disturbances
INTRODUCTION
Acid-base disturbances are broadly categorized as respiratory or metabolic in nature. Conventional acid-base analysis is based on the Henderson-Hasselbalch equation (Box 59-1), which uses bicarbonate concentration, [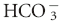 ], as the sole measure of the metabolic contribution to acid-base balance and the PCO2 as the single causative agent for respiratory acid-base disturbances. Using this conventional approach the primary abnormality (metabolic or respiratory) is the system that is responsible for the initial change in pH. For example, an acidemia with a low
], as the sole measure of the metabolic contribution to acid-base balance and the PCO2 as the single causative agent for respiratory acid-base disturbances. Using this conventional approach the primary abnormality (metabolic or respiratory) is the system that is responsible for the initial change in pH. For example, an acidemia with a low 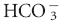 and low PCO2 would be considered a primary metabolic acidosis, because the acidemia must be a result of the low
and low PCO2 would be considered a primary metabolic acidosis, because the acidemia must be a result of the low 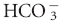 .1,2 The conventional approach identifies four acid-base abnormalities; these are described in Table 59-1.
.1,2 The conventional approach identifies four acid-base abnormalities; these are described in Table 59-1.
The PCO2 is universally considered the sole causative agent for respiratory acid-base disturbances. However, the parameter(s) used to assess metabolic disturbances varies with the analytic approach chosen. For clinical application, acid-base disturbances should be studied from the perspective of the individual etiologic agents that cause them (Table 59-2).
Table 59-2 Acid-Base Disturbances Based on Etiology
| Respiratory Causes of Acid-Base Disturbances | |
| Increased carbon dioxide | Respiratory acidosis (hypoventilation) |
| Decreased carbon dioxide | Respiratory alkalosis (hyperventilation) |
| Metabolic Causes of Acid-Base Disturbances | |
| Decreased sodium | Hyponatremic metabolic acidosis |
| Increased sodium | Hypernatremic metabolic alkalosis |
| Increased chloride | Hyperchloremic metabolic acidosis |
| Decreased chloride | Hypochloremic metabolic alkalosis |
| Increased protein | Hyperproteinemic metabolic acidosis |
| Decreased protein | Hypoproteinemic metabolic alkalosis |
| Increased phosphate | Hyperphosphatemic metabolic acidosis |
| Decreased phosphate | Hypophosphatemic metabolic alkalosis |
| Increased unmeasured anions | Lactic acidosis |
| Ketoacidosis | |
| Toxic acidosis (ethylene glycol, salicylate) | |
| Acidosis of renal failure | |
RESPIRATORY ACID-BASE DISTURBANCES
Conventional acid-base nomenclature states that a metabolic disturbance that is accompanied by an appropriate compensatory respiratory change is classified as a simple metabolic disturbance and the respiratory component is not named. A compensatory change is considered “appropriate” if it is of a magnitude similar to that predicted by the calculated compensatory response. These calculations are provided in Table 59-3 and are discussed further later in this chapter.1,3
Table 59-3 Expected Compensatory Changes to Primary Acid-Base Disorders
| Primary Disorder | Expected Compensation |
|---|---|
| Metabolic acidosis | ↓ PCO2 of 0.7 mm Hg per 1 mEq/L decrease in [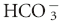 ] ±3 ] ±3 |
| Metabolic alkalosis | ↑ PCO2 of 0.7 mm Hg per 1 mEq/L decrease in [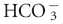 ] ±3 ] ±3 |
| Respiratory acidosis—acute | ↑ [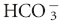 ] of 0.15 mEq/L per 1 mm Hg ↑ PCO2 ±2 ] of 0.15 mEq/L per 1 mm Hg ↑ PCO2 ±2 |
| Respiratory acidosis—chronic | ↑ [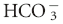 ] of 0.35 mEq/L per 1 mm Hg ↑ PCO2 ±2 ] of 0.35 mEq/L per 1 mm Hg ↑ PCO2 ±2 |
| Respiratory alkalosis—acute | ↓ [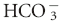 ] of 0.25 mEq/L per 1 mm Hg ↓ PCO2 ±2 ] of 0.25 mEq/L per 1 mm Hg ↓ PCO2 ±2 |
| Respiratory alkalosis—chronic | ↓ [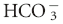 ] of 0.55 mEq/L per 1 mm Hg ↓ PCO2 ±2 ] of 0.55 mEq/L per 1 mm Hg ↓ PCO2 ±2 |
[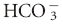 ], Bicarbonate concentration measured in mEq/L; PCO2, partial pressure of carbon dioxide measured in mm Hg, ↑, increased; ↓, decreased.
], Bicarbonate concentration measured in mEq/L; PCO2, partial pressure of carbon dioxide measured in mm Hg, ↑, increased; ↓, decreased.
Increased Carbon Dioxide: Respiratory Acidosis
Therapy for pathologic respiratory acidosis involves improving ventilation by treating the underlying cause. In severe cases of hypoventilation that persists despite therapy, mechanical ventilation is indicated (see Chapter 213, Basic Mechanical Ventilation). Elevated levels of CO2 can cause hypoxemia in patients breathing room air, and all animals with significant hypercapnia (>60 mm Hg) should receive oxygen therapy.4
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


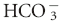 , base excess (BE), and anion gap, and the Stewart approach, which uses pH, PCO2, strong ion difference (SID), and the quantity of weak acids (ATOT). As clinicians, we find ourselves naming acid-base disturbances while gaining little understanding of the etiology of the patient’s acid-base problems and little insight into effective therapy. The primary problem is that within each of these traditional approaches multiple etiologic factors are grouped together. The clinician needs a way to isolate cause and effect to assess the mechanisms behind the disturbance and to focus treatment.
, base excess (BE), and anion gap, and the Stewart approach, which uses pH, PCO2, strong ion difference (SID), and the quantity of weak acids (ATOT). As clinicians, we find ourselves naming acid-base disturbances while gaining little understanding of the etiology of the patient’s acid-base problems and little insight into effective therapy. The primary problem is that within each of these traditional approaches multiple etiologic factors are grouped together. The clinician needs a way to isolate cause and effect to assess the mechanisms behind the disturbance and to focus treatment.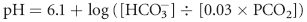
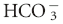 ] is the concentration of
] is the concentration of 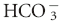 measured in mmol/L; 0.03 is the solubility coefficient for carbon dioxide in plasma; and PCO2 is the partial pressure of carbon dioxide in mm Hg.
measured in mmol/L; 0.03 is the solubility coefficient for carbon dioxide in plasma; and PCO2 is the partial pressure of carbon dioxide in mm Hg.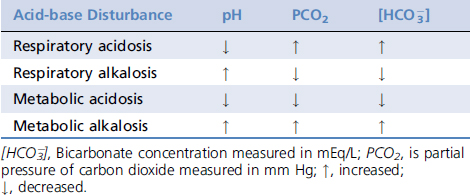
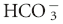 buffer system and ultimately the hydrogen ion concentration.
buffer system and ultimately the hydrogen ion concentration.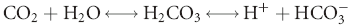
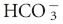 of 1 mEq/L should result in an increase in PCO2 of 0.7 mm Hg in both dogs and cats.1,3
of 1 mEq/L should result in an increase in PCO2 of 0.7 mm Hg in both dogs and cats.1,3