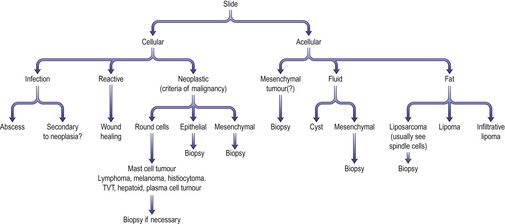
Cytology
Cytology is a quick, usually simple and inexpensive method of screening ‘lumps and bumps’. It is indicated for most palpable external masses and is useful for some internal masses or generalized diffuse organ changes with the assistance of ultrasound. It is also a valuable tool for examining effusions in body cavities, ‘washes’ (e.g. bronchoalveolar lavages (BAL), prostatic washes) and cerebrospinal fluid (CSF) analysis. Although cytology is useful in differentiating between tumour and non-tumour (e.g. infectious/inflammatory processes), when examining slides for suspected neoplasia it is important to remember that certain tumour cells generally exfoliate better than others, i.e. round cells > epithelial > mesenchymal. Impression smears taken from biopsy samples are also useful intra-operatively, especially if the type of lesion may affect the procedure and the ability to examine frozen sections is not available.
Slide preparation
The collection of cells and preparation of the slide are important as cells can be damaged and distorted when making preparations, making interpretation difficult or impossible (Villiers & Dunn 1998). Two techniques for obtaining samples from solid masses are used. In the first technique a needle is attached to a syringe (usually 5 or 10 ml), the needle is placed in the structure to be analysed and suction applied by drawing air into the syringe with the needle in the tissue. The aspirated tissue is expelled onto a clean slide, smeared using a second slide and stained. The second technique is known as fine-needle capillary sampling and involves simply placing the needle with no syringe into the tissue, usually two or three times; cells are displaced into the cylinder of the needle and are then expelled onto a slide as before. The latter technique may reduce the possibility of blood contamination and reduce damage to fragile cells.
Staining the slide
Most veterinary practices routinely use Romanowsky-type stains for staining in-house slide preparations. It is important to remember that the length of time for ‘dipping’ depends on the age of the stain (the stains should be changed regularly to ensure good results) and the cellularity of the preparation. Stains should be protected from light, kept covered when not in use and should be filtered periodically to eliminate debris and possible contaminants.
Examining the specimen
Once the specimen has been prepared, stained and dried, it should be examined systematically (Figure 4.1). Scan the whole slide under low power and then focus in on areas of interest. Remember that more than one process may be occurring at one time – for example, a tumour may have a secondary inflammatory or infectious component associated with it.
The initial step is to decide whether or not the lesion is inflammatory or non-inflammatory. Tumours are seen more frequently in middle-aged to older animals, whereas inflammatory lesions can occur at all life stages. Investment in a good cytology text is important for gaining experience in identifying neoplastic and non-neoplastic cytology (Baker & Lumsden 2000).
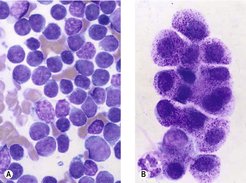
The major characteristic of active inflammation is the presence of the segmented neutrophil (Figure 4.2A). This can occur either as a consequence of infectious or non-infectious causes. Progression of inflammation to the chronic phase is characterized by the presence of macrophages in addition to neutrophils (Figure 4.2B,C). In certain cases it can be difficult to differentiate between macrophages (histiocytes) and neoplastic cells; if there is any doubt, a fresh unstained sample should be submitted for review by a clinical pathologist.
 |
| Figure 4.2 (Courtesy K. Freeman.) |
Remember inflammation/infection can be a component of neoplasms so for ulcerated cutaneous lesions a biopsy is recommended. The clinical history should always be considered in conjunction with the specimen.
Once inflammatory lesions have been ruled out, non-inflammatory causes should be considered.
Cytological preparations that would be non-neoplastic include ‘cystic’ structures, normal structures (e.g. fat, salivary glands) (Figure 4.2D) and hyperplastic tissue (reactive fibroblasts in recent scar tissue).
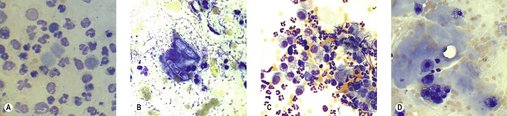
Neoplastic tissue, by definition, means ‘new growth’ so the criteria for defining potential neoplasia on cytology are cell type and the characteristics of malignancy. Caution must be applied when using the latter criterion on cytological specimens as hyperplasia (reactive features) and neoplasia can appear cytologically very similar – for example, it can be very difficult to differentiate between reactive mesothelial cells and neoplastic epithelial cells. If you are sure that the sample you are examining is from a tumour, the next question to resolve is whether or not it is benign or malignant. Common benign tumours include histiocytoma and perianal adenoma (Figure 4.3).
 |
| Figure 4.3 (Courtesy K. Freeman.) |
The criteria of malignancy to look for when examining a cytological preparation are outlined in Box 4.1.
Box 4.1
• Asymmetric mitotic figures
• Multinucleation
• Abnormal chromatin clumping/distribution
• Anisocytosis (variable cell size)
• Pleomorphism (variable shape)
• Anisokaryosis (variable nuclear cell size)
• Variable nucleolar size, shape or multiple nucleoli
• Changes in nuclear to cytoplasmic ratio
What is the next step?
If you are sure your sample is representative and you have ruled out the above non-neoplastic options, then it is important to characterize the type of tumour.
Tumours are classified into four broad categories:
• ‘round cell’ tumours
• epithelial cell tumours
• mesenchymal cell tumours
• neuroendocrine tumours.
‘Round cell’ tumours
These cells exfoliate very well and are characterized by being ‘round’ in appearance and appearing on a slide as single cells. Common round cell tumours that can be identified on cytology include lymphoma, plasma cell tumour, histiocytoma, mast cell tumour, melanoma, transmissible venereal tumour (TVT), hepatoid tumour (also may appear epithelial) and anaplastic tumours of any type (Figure 4.4).
Epithelial cell tumours
Epithelial cells exfoliate well and are typified by forming ‘rafts’ of cells with definite borders between cells because of the presence of tight junctions giving the appearance of a cobblestone pattern. Sometimes glandular and papillary configurations can be recognized.
Mesenchymal cell tumours
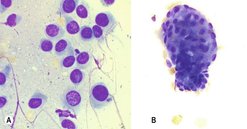
Cells of mesenchymal origin do not, as a rule, exfoliate well. Often a low yield of cells on an aspirate can be suggestive of mesenchymal origin (e.g. a sarcoma). On cytology these cells often have indistinct cell borders so it is difficult to delineate the end of the cytoplasmic border of one cell and the beginning of the next cell. The individual cells are elongated with central or eccentric nuclei (Figure 4.5).
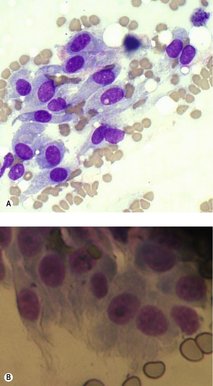 |
| Figure 4.5 Mesenchymal cell cytology (original magnification 500×). (A) Soft tissue sarcoma; (B) Osteosarcoma. (Courtesy K. Freeman.) |
It is important to remember that cytology is an important diagnostic tool but can on occasions be misleading, as sometimes it is not possible to differentiate cell type. A biopsy is usually the more accurate diagnostic tool in such circumstances.
Neuroendocrine tumours
These relatively rare tumours are characterized by possessing round to oval nuclei, often located within a ‘sea’ of cytoplasm. Some discrete cells may have granular or finely vacuolated cytoplasm and features of malignancy may be very subtle.
Cytological evaluation of lymph nodes
Evaluation of regional lymph nodes is an essential part of staging and, depending on the tumour type (mast cell tumours, epithelial tumours, melanomas), the lymph nodes can be the first site of dissemination. Sarcomas more typically spread haematogenously, but aggressive sarcomas can also involve the regional lymph nodes. Although fine needle aspirates (FNAs) are the backbone of staging from regional lymph nodes, it is important to remember that in equivocal cases excisional biopsies are required; palpation alone is inadequate to assess the possibility of metastatic spread (Langenbach et al 2001). Whenever possible the sentinel node should be removed at the time of surgery, irrespective of the results of cytology.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree