The treatment of cancer usually is a multimodality approach, potentially involving surgery, chemotherapy, radiotherapy or a combination of two or more of the above.
In order to select the correct treatment options for each patient it is important to know the following:
• Histological diagnosis
• Stage of malignancy for which treatment is being considered
• Any concurrent medical problems.
For each patient a minimum database is required:
• Blood work (biochemistry and haematology)
• Urinalysis
• Radiographs as indicated.
For full staging, other diagnostics may be necessary, e.g. ultrasound/contrast studies, and the appropriate diagnostics will be discussed in specific chapters. Defining the stage is imperative in being able to predict prognosis. In addition, concurrent disease may influence the oncologist’s choice of drugs, e.g. the use of lomustine in patients with liver disease. Once a diagnosis has been made it is important to define the goals and expectations of treatment.
Goals and expectations of treatment
Remission versus palliation
In many veterinary patients the goal is to achieve a complete remission, typically in patients with lymphoma. It is important to realize that a complete remission means that there is no visible sign of the tumour, not that all the cancer cells have been eliminated. This means that at some point the cancer will recur and, when it does, drug resistance may have occurred. In some cases (e.g. osteosarcoma), the goal is to prolong median survival time whilst informing the client that eventually the cancer will return in the form of metastatic disease. When chemotherapy is used in this fashion it is known as adjuvant therapy and treatment is targeted at micrometastatic disease. The third situation is where chemotherapy is being used to shrink a tumour in an attempt to improve quality of life without any possibility of a remission (e.g. large metastatic mast cell tumours) and is described as palliative therapy. In some instances chemotherapy may be used in an attempt to shrink a tumour prior to surgery (neoadjuvant treatment).
Expected and potential side effects of treatment
It is the responsibility of the veterinary surgeon to fully understand the potential side effects of the drugs they intend to administer and when taking on that responsibility ensure that they have the facilities to provide adequate 24-hour care. Side effects can be acute (vomiting/diarrhoea), cumulative (bone marrow suppression) or idiosyncratic (haemorrhagic cystitis with cyclophosphamide).
Quality of life for the patient
It is the goal of every veterinary oncologist to give the cancer patient the greatest quantity of life without compromising the quality of that life or the bond between the client and their beloved companion.
Realistic expectation as to survival time for an individual
Statistics on median survival times for the most common veterinary cancers are known, e.g. lymphoma (see Chapter 22) and osteosarcoma (see Chapter 21). With some cancers that occur less frequently a lot of data is unavailable, and the expertise and experience of the clinical oncologist are required to assess the balance between risk and benefit of chemotherapy in the individual. It is important to remember that every patient is an individual and that many factors can influence overall survival times, not least being signalment, stage of disease, concurrent medical problems and the protocol selected.
What are the indications for the use of chemotherapy?
Chemotherapy is primarily given systemically in small animals. The major indications are:
• most effective single therapy for some malignancies, e.g. lymphoma and leukaemia (however, in certain cases, radiotherapy may be the treatment of choice)
• adjuvant treatment for highly metastatic tumours after surgery, e.g. osteosarcoma (OSA)
• shrinkage of large tumours prior to surgery or to relieve pain due to the large size of a tumour, when no other treatment is considered or practical (invariably, radiotherapy is the superior option in such cases)
• radiation sensitization – certain chemotherapeutics act synergistically with radiation to improve cell kill.
Rarely in veterinary medicine chemotherapy will be given intralesionally (Yoshida et al 1998) or intracavitary (Moore et al 1991) for tumours where systemic administration is unlikely to result in a high enough concentration of drug reaching the target.
Once the decision to treat has been made, the next step is selection of protocol. There are a number of cytotoxic drugs used in the treatment of canine and feline cancers and more and more drugs are becoming available to the veterinary oncologist. Selection of appropriate drugs depends on species, type of cancer, availability, experience of the veterinary surgeon, facilities to handle these drugs and the cost.
Many sources are available to obtain dosages for the better-known drugs. It is important that drugs are administered at the optimal time interval. If they are given too close together then significant toxicity can result, especially if the toxicities of the drugs overlap. Alternatively, if the drugs are given too far apart, tumour cells have time to develop resistance and repopulate. It is known that the repair enzymes of tumour cells are not as efficient as those of normal cells, and, therefore, it is possible to capitalize on this by scheduling treatments such that normal cells have had time to repair but the cancer cells have not. However, it is important that the patient is well enough to receive treatment; if in any doubt, a short delay may be necessary.
What are the principles on which chemotherapeutic protocols are derived?
Combination protocols
Combinations of drugs provide maximum tumour cell kill whilst limiting individual drug toxicity. Utilization of drugs that have different mechanisms of action means that resistance within a heterogeneous population will develop more slowly. Tumours that are large enough to be detected (>106 cells) are a heterogeneous population containing drug-resistant clones (Goldie et al 1982). This means that with single-agent protocols response will be short because of the rapid repopulation with resistant cells.
Drug selection is based on proven efficacy of the drug against the tumour in question and the ability to give it at consistent intervals; the treatment-free interval between cycles should be the shortest time possible for the recovery of the most sensitive normal tissue, usually the bone marrow. The storage compartment of the bone marrow is able to supply mature cells for ~10 days after the stem cell pool has been damaged. This means that what is seen in the peripheral blood is about a week behind what is going on in the marrow.
The nadir of leucopenia exhibited by the various drugs is an important consideration in chemotherapy protocols. All drugs should be given at their optimal dose and schedule. When several drugs of a class are available, the drug selected should not have overlapping toxicities with other selected drugs. In some instances, however, single agents are used as the overall survival times with combination protocols have not been shown to be superior to single agents but the toxicity is significantly greater, e.g. doxorubicin in the management of splenic haemangiosarcoma (see Chapter 23).
Fractional kill hypothesis and tumour cell number
There is an inverse relationship between the total number of neoplastic cells and ‘curability’. Early diagnosis and early treatment give the patient the best possible prognosis. The same percentage of cells is killed per cycle of a given drug, irrespective of the number of cells. This is known as the fractional kill hypothesis and each cycle of treatment will kill a specific fraction of the remaining cells. The objective of each cycle is to reduce the absolute number of remaining cells due to the cumulative effect of successful fractional kills.
Pharmacological principles
The pharmacokinetics of chemotherapeutic agents is important as this examines the distribution of drugs and their metabolites throughout the body and ultimately the bioavailability of drugs to kill cancer cells and therefore their therapeutic effect (Ehrlichman 1992). Intravenous administration assumes 100% bioavailability but administration via other routes (oral, intramuscular, subcutaneous) may only be partial. It is important to remember that the bioavailability of many chemotherapeutic drugs used in veterinary oncology that are not given i.v. will not have had their bioavailability validated.
A number of factors influence the distribution of drugs within the body and include blood flow to different organs, diffusion of drug from blood vessels, protein binding and lipid solubility. Doxorubicin shows extensive tissue binding and therefore has slow release of the drug from these sites. A drug such as carmustine (BCNU) that is lipid soluble, and therefore penetrates the blood–brain barrier, should also have a slow elimination phase; however, it is inactivated quickly, meaning that there is only a very short exposure of body tissues to the drug.
Peak plasma concentration versus concentration over time
The cytotoxic effect depends on concentration over time, whereas toxicity to normal tissue depends on peak plasma concentration.
Therapeutic index
Therapeutic efficacy is based on giving the maximum amount of drug that causes minimal toxicity. For most chemotherapeutics the margin between therapeutic dose and toxicity is narrow and care must be taken to administer these drugs accurately. Drug dosages are usually calculated as mg/m2 or mg/kg (see Table 6.8 and Table 6.9 at the end of this chapter). When calculating doses using the m2 chart, it is important to remember that a small dose reduction may be required in small dogs and in older patients where the bone marrow is not as resilient.
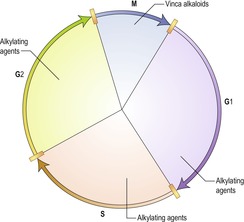
Cell cycle
The cell cycle (Figure 6.1) is important when putting together a chemotherapy protocol and drugs can be broadly classified into those that affect a particular point in the cell cycle, known as cell-cycle specific, e.g. the vinca alkaloids, and those that can act on the cell at any time during the cell cycle, non-cell cycle specific, e.g. alkylating agents.
Classification of drugs
Chemotherapeutics are classified both by their mechanism of action and their chemical structure. A discussion of some of the cytotoxic drugs most commonly used in veterinary medicine follows; this is by no means an exhaustive list and for further details concerning these drugs and other cytotoxics not listed here, reference to a more advanced text is recommended. Table 6.1A, Table 6.1B, Table 6.1C, Table 6.2, Table 6.3, Table 6.4, Table 6.5 and Table 6.6 describe the major groups of drugs, the most frequently used members of the group, known mechanism of action, method of excretion and some of the more commonly seen side effects; this is not a complete description of the drugs but rather a guideline.
| Cyclophosphamide (Endoxana, Cytoxan) | Chlorambucil (Leukeran) | Melphalan (Alkeran) | Ifosfamide | |
|---|---|---|---|---|
| Mechanism of action | Alkylation of DNA | Alkylation of DNA | Alkylation of DNA | Alkylation of DNA |
| Metabolism | Microsomal hydroxylation to active form, hydrolysis to acrolein, excretion as inactive products | Chemical decomposition to active and inactive products | Chemical decomposition to inert products | As other alkylating agents |
| Principal toxicities | Myelosuppression, platelets spared, sterile haemorrhagic cystitis, alopecia | Platelets affected | Myelosuppression (delayed nadir 4–6 weeks) | Myelosuppression Severe haemorrhagic cystitis can result with this drug – uroprotect with 2-mercaptoethanesulfonate (MESNA) |
| Precautions | Tablets should not be divided Appropriate handling of lyophilized powder within a safe environment is necessary | Tablets should not be divided | Tablets should not be divided | As for other alkylating agents |
| Drug dosage | Dogs: 200–250 mg/m2 Cats: 10 mg/kg | Dogs: 0.1–0.2 mg/kg once daily (individual protocols vary, so check protocol carefully before prescribing) Cats: 2 mg every other day | Dogs: 0.1 mg/kg daily for 10 days then 0.05 mg/kg daily | Dogs: 350–375 mg/m2 i.v. q 3 weeks |
| Indications | Lymphoma, leukaemia, multiple myeloma, plasmacytomas | Chronic lymphocytic leukaemia, lymphoma, multiple myeloma | Multiple myeloma, lymphoma | No proven advantage over cyclophosphamide Sterile haemorrhage cystitis is a concern |
| CCNU (Lomustine, CeeNU) | BCNU (Carmustine) | Busulphan | |
|---|---|---|---|
| Mechanism of action | Nitrosourea alkylates DNA and RNA Not cross resistant with other alkylating agents | Nitrosourea Alkylates DNA as lomustine | Alkylating agent |
| Metabolism | Metabolized by the liver Active metabolites excreted via kidneys | As for lomustine | Metabolized to several products and excreted in the urine |
| Principal toxicities | Myelosuppression can be severe especially at higher doses and is dose limiting Hepatic, renal | Myelosuppression can be severe Nausea, vomiting | Bone marrow suppression (myelosuppression and thrombocytopenia) can be prolonged |
| Precautions | Consider dose reduction in patients with hepatic/renal disease | Associated with pulmonary fibrosis in humans | Tablets should not be divided See also other alkylating agents |
| Drug dosage | Dogs: 60–90 mg/m2 p.o. Cats: 50 mg/m2 p.o. | Dogs: 50 mg/m2 i.v. every 6 weeks | Dogs: 2 mg/m2 p.o. |
| Indications | Lymphoma, mast cell tumours, histiocytic sarcomas in combination with radiotherapy, and gliomas | Rarely used in veterinary medicine, but does cross the blood–brain barrier | Chronic myelogenous leukaemia |
| Drug | Procarbazine (Matulane) | Dacarbazine (DTIC) |
|---|---|---|
| Mechanism of action | Alkylation of DNA | Exact mechanism is unknown but at least in part acts as an alkylating agent |
| Metabolism | Metabolised by the liver, excreted by the kidney as inactive metabolites | Drug is eliminated by renal tubular secretion and extensively metabolized in the liver |
| Principal toxicities | Leucopenis and thrombocytopenia-late onset | Myelosuppression, hepatotoxicity, anorexia, nausea, vomiting, alopecia, anaphylaxis |
| Precautions | Oral-capsules should not be divided | Vesicant if extravasated. Incompatible with hydrocortisone sodium succinate |
| Drug dosages | Dogs 50 mg/m2 daily for 14 days (MOPP protocol) | Dogs 200 mg/m2 iv as a slow bolus. Day 1–5 or every 3 weeks in combination with doxorubicin or CCNU |
| Possible Indications | Rescue protocols for lymphoma | Rescue protocols for lymphoma |
| Vincristine (Oncovin) | Vinblastine | Vinorelbine | |
|---|---|---|---|
| Mechanism of action | Inhibits polymerization of tubulin | Inhibits polymerization of tubulin | Semisynthetic derivative of vinblastine |
| Metabolism | Hepatic with biliary excretion | Hepatic with biliary excretion | As with other vinca alkaloids |
| Principal toxicities | Ileus, constipation, peripheral neuropathy | Neutropenia, thrombocytopenia | Neutropenia; peripheral neuropathy greater than vincristine, constipation |
| Precautions | Vesicant if extravasated | Vesicant – care with catheter placement | As for other vinca alkaloids |
| Drug dosage | Dogs: 0.5–0.75 mg/m2 Cats: 0.025 mg/kg or 0.75 mg/m2 | Dogs and cats: 2 mg/m2 as per protocol | Dogs: 15–18 mg/m2 i.v. weekly |
| Indications | Leukaemias, lymphomas, some solid carcinomas and sarcomas, mast cell tumours | Lymphomas, leukaemias, mast cell tumours | Rarely used in veterinary medicine |
| Doxorubicin (Adriamycin) | Epirubicin | Mitoxantrone (Novantrone) | Actinomycin D (Cosmegan, Dactinomycin) | |
|---|---|---|---|---|
| Mechanism of action | Pleiotropic effects: • inhibition of DNA topoisomerase II activity • free radical damage • apoptosis and others | Pleiotropic effects: • inhibition of DNA topoisomerase II activity • free radical damage and others | Inhibits topoisomerase II | Antitumour antibiotic |
| Metabolism | Doxorubicinol major metabolite 50–60% of parent drug accounted for by known routes of elimination, mostly biliary Can bind to DNA and cardiolipin in tissue and is slowly dissociated | Primarily parent compound drug accounted for by known routes of elimination, mostly biliary | Primarily excreted by the liver | Excretion through urine and faeces |
| Drug interactions | Binds heparin and forms aggregates | Incompatible with heparin | Incompatible with heparin | – |
| Principal toxicities | Myelosuppression (nadir 7–10 days), gastrointestinal, alopecia, cardiac Severe local tissue damage if extravasated Allergic reactions | As for doxorubicin More gastrointestinal side effects have been seen with epirubicin | Myelosuppression (nadir 7–10 days) Fewer gastrointestinal and allergic reactions | Myelosuppression, gastrointestinal (nausea and vomiting) |
| Precautions | Ensure i.v. catheter is securely placed to prevent extravasation Dose-related cardiomyopathy, radiation recall, possible dose reduction with severe hepatic disease | Vesicant if extravasated | Mild vesicant | If extravasated will cause pain, swelling and necrosis |
| Drug dosage | Dogs: 30 mg/m2 every 3 weeks Cats: 1 mg/kg every 3 weeks | As with doxorubicin | Dogs: 5.0–5.5 mg/m2 every 3 weeks Cats: 5.0–6.5 mg/m2 every 3–4 weeks | Dogs: 0.5–0.7 mg/m2 |
| Indications | Leukaemias, lymphomas, carcinomas (mammary), sarcomas (haemangiosarcoma) – wide range of activity | Substitute for doxorubicin Cardiac toxicity has been reported and this drug should not be used in patients with pre-existing heart conditions | Squamous cell carcinoma, lymphoma, transitional cell carcinomas Substitute for doxorubicin | Salvage drug for patients with lymphoma May be beneficial for patients with acute lymphoblastic leukaemia |
| Cisplatin (Platinol) | Carboplatin (Paraplatin) | |
|---|---|---|
| Mechanism of action | Covalently binds to DNA | Covalently binds to DNA |
| Metabolism | Inactivated intracellularly Elimination: 26% excreted in first 24 hours; >90% renal excretion | 90% excreted in urine in 24 hours |
| Principal toxicities | Nephrotoxic, nausea/vomiting, myelosuppression, ototoxicity, peripheral neuropathy, hypersensitivity, seizures Pulmonary oedema in cats | Myelosuppression, nausea/vomiting Possible nephropathy in patients with prior renal disease |
| Precautions | Aggressive diuresis and anti-emetic therapy necessary Maintain high urine flow during therapy Monitor BUN/creatinine and urine for casts before each treatment Do not administer with other nephrotoxic drugs (e.g. NSAIDS or aminoglycosides) | Diuresis is recommended before treatment in patients with history of renal disease Monitor renal parameters |
| Drug dosage | Dogs: 50–70 mg/m2 Fatal in cats | Dogs: 300 mg/m2 Cats: 180–210 mg/m2 |
| Indications | Osteosarcoma, intracavitary mesothelioma, carcinomatosis, squamous cell carcinoma Radiosensitizer for nasal tumours | Osteosarcoma, squamous cell carcinoma Intracavitary, radiosensitizer |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree