Tumours of the thyroid
Thyroid tumours account for approximately 1–4% (Birchard & Roesel 1981, Loar 1986, Ogilvie 1996, Priester & McKay 1980, Waters & Scott-Moncrieff 1998, Wheeler 1989) of all canine tumours, and are typically seen in middle-aged to older dogs, with a median age of 9–10 years (Brodey & Kelly 1968, Harari et al 1986, Sullivan et al 1987). Breeds over-represented include the Boxer, Beagle and Golden Retriever (Harari et al 1986). Aetiology is unknown.
Most dogs with thyroid carcinoma are clinically euthyroid, and 55–60% have normal thyroid hormone concentrations (Feldman & Nelson 2004). Low serum thyroid hormone concentrations (hypothyroidism) are seen in 30–35% (Feldman & Nelson 2004) and may be due to destruction of the gland by the tumour, or possibly from pre-existing hypothyroidism, or from large thyroid tumours that produce significant amounts of inactive thyroid hormone which may suppress thyroid-stimulating hormone (TSH) secretion and lead to atrophy of the normal thyroid gland (Loar 1986). Only 10%–20% are functional (hyperthyroid) (Brodey & Kelly 1968, Feldman & Nelson 2004).
Thyroid adenomas are also seen (30–50% of thyroid tumours). They are usually small, non-functional and detected incidentally at post-mortem, but have been reported to reach >6 cm in size (Brodey & Kelly 1968, Leav et al 1976).
Thyroid carcinomas are classified as either follicular (solid or mixed) or parafollicular (medullary or C cell). Follicular thyroid carcinomas may be very large and invasive. These tumours are malignant, with metastasis found in 16–60% of patients at the time of initial diagnosis (Carver et al 1995, Harari et al 1986, Jeglum & Whereat 1983, Kent et al 2002, Withrow & McEwen 2001), and 60–80% of patients will ultimately develop metastatic disease at necropsy (Capen 1985, Leav et al 1976, Verschueren et al 1992). Metastasis is generally to regional lymph node and lungs (Brodey & Kelly 1968, Leav et al 1976). Other metastatic sites include adrenal gland, brain, heart, kidneys, liver and bone.
Thyroid carcinoma can also arise from ectopic tissue in the tongue, ventral neck and cranial mediastinum. Thirty-three per cent of thyroid carcinomas are freely mobile, without invasion into surrounding tissue; 67% are fixed, with dyspnoea, dysphonia, dysphagia and Horner’s syndrome as clinical signs. Approximately one-third are bilateral (Feldman & Nelson 2004).
Clinical signs
In many cases thyroid tumours are not noticed by the client until they are extremely large and present to the veterinary surgeon for a mass on the ventral neck that is non-painful and not causing the dog any obvious discomfort. When questioned, the client may have noticed a change in phonation prior to presentation. Other clinical signs seen less frequently include dysphagia, dyspnoea and Horner’s syndrome.
Diagnostic work-up
Fine needle aspiration (FNA) cytology can show malignant epithelial cells, with blood contamination common. A cervical mass with a bloody FNA is suspicious of thyroid carcinoma; the other differentials include neuroendocrine tumours or haemangiosarcoma. There is no need to perform an incisional biopsy preoperatively for a unilateral mobile neck mass if the FNA is highly suspicious of thyroid carcinoma, especially as this may greatly complicate surgical excision because the biopsy tract must be removed en bloc with the tumour. The purpose of an FNA is to differentiate a suspected thyroid mass from other causes of swelling in this area.
A definitive diagnosis requires a biopsy, which should be excisional if the tumour is surgically excisable (as judged by an experienced surgeon) or incisional if the tumour is fixed, bilateral or deemed not to be surgically excisable. The surgeon is warned that these tumours are extremely vascular (particularly when fixed and invasive) and biopsy or excision may cause significant haemorrhage. Even a small surgical procedure can result in considerable blood loss due to the highly vascular nature of these tumours.
An ultrasound of the neck may be beneficial for diagnosis (e.g. identification of a mass of thyroid origin, ultrasound-guided FNA, assessing tumour vascularity) and may also be used to examine the regional (e.g. cervical and retropharyngeal) lymph nodes for metastasis (Wisner & Nyland 1998, Wisner et al 1994). However, a CT or MRI scan is of much greater value in these cases.
Evaluation of T4 may be useful to check thyroid status but is rarely of clinical significance. Scintigraphy, when available, may be useful as an abnormal accumulation of sodium pertechnetate (Tc99m) may also assist in the diagnosis of ectopic thyroid malignancy. The functional capability of normal thyroid should be determined as part of the diagnostic work-up and as part of follow-up evaluation (Marks et al 1994). Iodine-131 (131I) uptake may also be useful for diagnosis of local tumour.
Staging
Regional lymph nodes should be palpated for enlargement and aspirated if suspicious. Haematology, serum biochemistry and urinalysis are routinely performed as part of a general health assessment. Survey thoracic radiographs are standard. A thoracic CT is more sensitive than thoracic radiographs to rule out disseminated disease. Cervical ultrasound can be useful, but for planning treatment of large fixed tumours a contrast-enhanced CT or MRI is required. Nuclear scintigraphy can be used for determining function and identifying ectopic malignant tissue. Scintigraphy is also useful for staging.
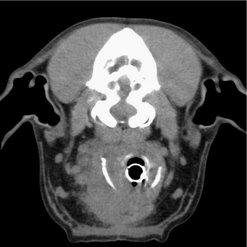
Differential diagnoses include abscess, granuloma, salivary mucocele, salivary adenocarcinoma, lymphoma, carotid body tumours (Figure 26.1), haemangiosarcoma or other sarcoma, and finally metastasis from oral squamous cell carcinoma (SCC).
Treatment
Options depend on the size, degree of invasion, clinical signs of hyperthyroidism and the availability of radiotherapy and nuclear medicine.
Surgery
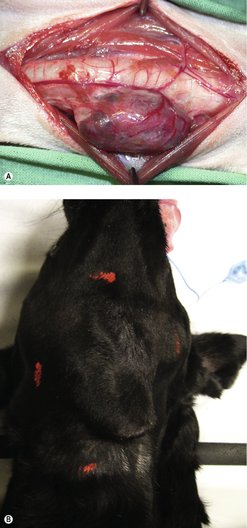
This is the treatment of choice for freely movable masses. It is important that an experienced oncological surgeon evaluates ‘movability’ as surgically excisable masses can seem fixed to the inexperienced surgeon. The surrounding trachea and neck muscles and deeper location of the neck mass can ‘hold’ the tumour in place and give a false idea of fixation (Figure 26.2). In the authors’ experience, if it is a rounded, well-circumscribed mass and is even slightly movable, it is likely to be surgically excisable. If it is large, invasive and totally fixed and immobile, it is not surgically excisable, and attempting to remove it would likely result in a great deal of haemorrhage. Some are very obviously freely mobile and these are most amenable to surgical excision.
Unilaterally, the carotid artery, jugular vein and vagosympathetic trunk can be sacrificed with minimal morbidity. Surgery can be complicated by local invasion and excessive haemorrhage or regional coagulopathy.
Prognosis with surgery
• Freely mobile: the prognosis is good with a median survival time (MST) of 36 months, a 1-year survival rate of 75% and a 2-year survival rate of 70% (Klein et al 1995).
• Fixed: with surgery alone the prognosis is poor with an MST of 10 months, a 1-year survival rate of 5% and a 2-year survival rate of 10% (Carver et al 1995).
Radiotherapy
Radiation has a number of applications in the management of thyroid carcinomas. It can be used as adjunctive therapy to reduce recurrence after incomplete resection or to shrink tumours down prior to surgery. In the latter instance it is the deep margin that is important to evaluate for making a non-surgical patient into a surgical one (see Figure 26.2B).
Radiotherapy is the treatment of choice for large non-resectable tumours. A number of different protocols have been used. Théon et al (2000) reported a progression-free interval of 80% at 1 year and 72% at 3 years using a hyperfractionated protocol. The metastatic rate was 28% and the MST was 24.5 months.
For dogs with non-resectable disease, a hypofractionated protocol (4 once-weekly fractions of 900 cGy) resulted in good long-term control with an MST of 24 months. A complete response was seen in 11% of patients, with most dogs typically having a partial response (89%). No difference in survival was noted between dogs with and without metastatic disease at the time of diagnosis, suggesting that metastatic lesions are very slow growing (Brearley et al 1999).
Chemotherapy
Overall the value of chemotherapy in the management of thyroid carcinomas is debatable. In one study a partial response was seen in 30–50% of dogs treated with either doxorubicin or cisplatin. Cisplatin 9% (1/11) had a complete response and 54% (6/11) a partial response. The overall MST was 98 days although the MST for responders was 322 days (Fineman et al 1998).
Iodine-131 (131I)
Responses to 131I have been reported but the number of cases is small. 131I is indicated for non-resectable thyroid carcinoma, incomplete resection of thyroid carcinoma and metastatic thyroid carcinoma (Worth et al 2005), but is rarely applicable in most cases because of radiation protection issues and the availability of resources. In a recent study of 131I therapy (± surgery) in 39 dogs with non-resectable thyroid tumours, prolonged survival times were achieved, regardless of serum thyroxine concentration prior to treatment, although three dogs died of bone marrow suppression. Dogs with local or regional tumours lived a median of 839 days; dogs with metastasis lived a median of 366 days (Turrel et al 2006). In another study, 11 dogs that received 131I adjunctively to surgery had an MST of 34 months, dogs receiving 131I alone had an MST of 30 months and dogs with no treatment had an MST of only 3 months (Worth et al 2005).
Prognosis
An early diagnosis, with a freely movable, small primary tumour, treated with complete surgical excision, correlates to a good to excellent prognosis. A delayed diagnosis allows further growth, invasion and fixation of the primary tumour, and a poorer overall prognosis.
Large tumours tend to be poorer candidates for surgery, and are more likely to have metastasized. Tumours >20 cm3 or >5 cm in diameter are at a higher risk of developing metastatic disease (Leav et al 1976).
If the tumour is non-resectable, the options for treatment are then considered to be palliative, i.e. radiotherapy (treatment of choice), chemotherapy or 131I.
Patients with bilateral thyroid carcinoma have a 16 times greater risk of developing metastatic disease (Théon et al 2000). In addition, patients with non-medullary thyroid carcinomas may be more likely to develop metastatic disease (Carver et al 1995).
Tumours of the parathyroid gland
Tumours of the parathyroid gland are uncommon and typically are benign (adenomas/hyperplasia). Carcinomas are seen less frequently (Berger & Feldman 1987). Typically older dogs are affected (age range 7–13 years) with no apparent sex predilection. Keeshonds may be over-represented (Goldstein et al 2007, Skelly & Franklin 2007, Weir et al 1986).
Clinical signs
These patients typically present with polyuria/polydipsia (PU/PD). Other non-specific signs include listlessness and muscle weakness (Berger & Feldman 1987). Calcium-based urinary calculi may also occur (DeVries et al 1993).
Diagnostic work-up
Any patient presenting for PU/PD should have a full biochemistry profile carried out, including total and ionized calcium. Serum calcium is either ionized (free, i.e. biologically active: 56%), chelated (bound to phosphate, bicarbonate, sulphate, citrate or lactate: 10%) or protein-bound (34%) (Schenck et al 1995). The identification of hypercalcaemia as the cause of PU/PD means an investigation into the underlying causes for hypercalcaemia.
Differential diagnoses include primary hyperparathyroidism, a number of neoplastic disorders including lymphoma, multiple myeloma and anal sac adenocarcinoma, and metabolic disorders including hypoadrenocorticism, vitamin D toxicity, renal failure and skeletal disorders. Laboratory error (lipaemia, haemoconcentration, haemolysis) can also cause hypercalcaemia. Elevations in serum albumin or globulins may elevate total serum calcium, but the free/ionized/biologically active calcium remains unchanged/normal.
As parathyroid gland tumours are rare, typically other causes of hypercalcaemia are ruled out first. Thorough physical examination, including a rectal examination, is essential. Thoracic and abdominal radiographs and abdominal ultrasound rule out other causes of hypercalcaemia. Other tests would include haematology, ACTH stimulation test and bone marrow evaluation. If no other cause of hypercalcaemia is identified, measurement of serum parathormone (PTH) and parathormone-related peptide (PTH-rP) is indicated. However, the improved availability of specific hormonal tests means that, in patients with a high index of suspicion for parathyroid gland tumours, PTH and PTH-rP should be assessed early in the diagnostic process.
Primary hyperparathyroidism occurs with elevated total serum calcium, normal to increased serum PTH, and increased ionized calcium.
Ultrasound of the thyroid and parathyroid glands can assist in diagnosis and aid the surgeon not only to localize the tumour but also to check for multiple tumours, as multiple parathyroid adenomas are not uncommon in dogs (DeVries et al 1993, Feldman & Nelson 2004). Eighty per cent of dogs have single gland involvement and 20% have multiple gland involvement (Arnaud & Kolb 1991). Although ultrasound findings correlate well with surgical findings (Gear et al 2005), in many cases cervical ultrasound may be negative. Exploratory surgery is indicated if primary hyperparathyroidism is suspected based on PTH and ionized calcium levels regardless of negative cervical ultrasound findings. Scintigraphy can also assist in diagnosis (Matwichuk et al 1996).
Treatment
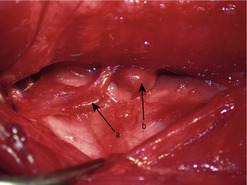
Preoperatively, hypercalcaemia can be managed with saline diuresis, but only until the patient is stable enough for surgery. The treatment of choice is prompt surgical excision of the affected parathyroid gland(s). Surgical exploration should be performed via a ventral midline approach, to allow for exploration and examination of each thyroid gland and the associated four parathyroid glands (Figure 26.3). All four parathyroid glands can be removed, although lifelong calcium and vitamin D supplementation will be required.
Enlargement of all four glands due to primary hyperparathyroidism is reported (DeVries et al 1993, Feldman & Nelson 2004); however, it is extremely rare and generally suggests a diagnosis other than hyperparathyroidism (i.e. secondary hyperplasia). As long as one of the parathyroid glands is not removed there is no risk of permanent hypocalcaemia.
Postoperative hypocalcaemia is a common short-term complication (usually within 1–4 days) after removal of a parathyroid tumour because of tumour-induced atrophy of the remaining glands, and management of serum calcium is critical until the remaining glands are functioning normally. Providing there has been no permanent damage to the kidneys from prolonged hypercalcaemia, the overall prognosis after surgery is good.
Treatment of parathyroid tumours using ultrasound-guided intralesional ethanol injections and radiofrequency heat ablation has been described (Long et al 1999, Pollard et al 2001). Intralesional ethanol injections appear the more successful of the two techniques.
Prognosis
In the rare cases of parathyroid carcinoma the prognosis is still good. Feldman & Nelson (2004) report that 5–10% of their canine cases of primary hyperparathyroidism were given a diagnosis of a parathyroid carcinoma on histology, but none had distant metastasis. This has also been the clinical experience of the authors, although one patient died of metastatic disease within 6 months. Non-malignant parathyroid tissue can have many of the histological features of malignancy and the surgeon is therefore justified in giving a cautiously optimistic prognosis for cases of parathyroid carcinoma that appear to have all the clinical features of parathyroid hyperplasia or adenoma. The role of radiotherapy or chemotherapy in patients with parathyroid carcinoma has not been explored, and would seem hard to justify given the previous comments.
For patients with persistent hypercalcaemia due to metastatic spread, medical management may provide some symptomatic relief. Diuresis with 0.9% NaCl should be instigated and when fully hydrated calcium-wasting diuretics (furosemide) may be used. It is important to ensure full hydration before using diuretics to prevent any further renal damage. Prednisolone is rarely beneficial but other agents, including calcitonin and bisphosphonates, may provide some symptomatic relief. The prognosis for the patient with metastatic spread is poor.
APUDomas
APUD stands for amine precursor uptake and decarboxylation. These tumours are of neuroendocrine origin and arise from specialized cells located in the pancreas, gastrointestinal tract, pituitary, thyroid and adrenal glands. APUD cells possess a neuron-specific enolase and the presence of this enolase can be used to identify these tumours histologically. Most APUDomas are of pancreatic origin and include insulinoma, glucagonoma and gastrinoma.
Pancreas
Insulinoma
Insulinoma is the most common tumour of the canine pancreas. It is extremely rare in the cat. The cell of origin is the pancreatic beta cells and the majority of insulinomas (80%) are functional carcinomas. Visceral metastases to the regional lymph nodes and liver are common.
Signalment
Insulinoma is seen in middle-aged to older dogs, with an increased incidence in German Shepherds, Irish Setters and Golden Retrievers (Caywood et al 1998). However, any breed of dog may be affected.
Clinical signs
These include weakness, lethargy, ataxia, seizures, muscle tremors, collapse, and ultimately coma. These are often episodic and are a consequence of hypoglycaemia that results in neuroglycopenia and increased catecholamine production. Peripheral neuropathies have also been reported (Braund et al 1987, Shahar et al 1985, Van Ham et al 1997).
Diagnostic work-up
Insulinoma should be suspected when there is a high serum insulin level and associated hypoglycaemia.
Ultrasound
Ultrasonography may be helpful, but many of these tumours are small and difficult to detect on ultrasound. In one study, ultrasound identified 75% of pancreatic neoplasias but was only 55% sensitive for detecting metastasis (Lamb et al 1995).
CT and scintigraphy
CT identified 10 of 14 pancreatic masses but overestimated metastasis (Robben et al 2005).
Scintigraphy and single photon emission CT with radiolabelled octreotide (somatostatin analogue) appear as good as ultrasound for detecting primary pancreatic neoplasia, but have poor sensitivity for detecting metastasis (Robben et al 2005). Robben et al (1997) reported visualization and localization of primary pancreatic tumour and/or metastasis in five of six dogs; multiple metastases (<3 mm) were found in the liver at necropsy in the remaining dog, apparently too small to be visualized by single photon emission CT. Radiolabelled pentetreotide (somatostatin analogue) scintigraphy accurately predicted the anatomical location of the primary tumour in one of four dogs (Garden et al 2005).
Although imaging modalities can be helpful for diagnosis and staging, a definitive diagnosis and accurate staging for prognosis require exploratory surgery and biopsy.
Surgery
The treatment of choice is partial pancreatectomy to remove the primary and, if possible, any metastatic lesions. Insulinomas are malignant, with most patients having microscopic or gross metastases at the time of surgery. In one study, 14 dogs with suspected gross metastases at surgery had this confirmed by biopsy in only 8 (Tobin et al 1999). The most common site of metastases is to the regional lymph nodes and liver.
In 92% of cases, an obvious mass was found in the pancreas at the time of surgery (Feldman & Nelson 2004). The primary tumour may be in either lobe (equal frequency) and up to 15% have multiple nodules (Caywood et al 1998, Tobin et al 1999). Feldman & Nelson (2004) reported 42% were in the right (duodenal) lobe, 41% in the left (splenic) lobe and 17% in the central region (body). Most tumours are visible but some are not, and are only found with gentle palpation of the pancreas. The surgeon should handle the pancreas very gently and carefully, as finding the tumour can be difficult once the pancreas has been traumatized and inflamed with repeated palpations. Minimal margins (1–2 mm) of grossly normal pancreas around the mass appear adequate, and there are no data to say whether or not taking a wider margin of grossly normal pancreas is helpful for survival or recurrence. Haemoclips, suture–fracture technique or TA-30 staples can be used to assist removal.
Intraoperative ultrasound has been used to identify small tumours (Robben et al 2005). If no tumour can be found in the pancreas, one option is to remove half of the pancreas and send it for histopathology, in the hope that tumour will be identified. If no discrete tumour is found, the pathologist should check for diffuse microscopic islet cell carcinoma (reported in 4 of 85 dogs). If the resected pancreas fails to show any abnormal tissue, the other half could then be removed, as only a small amount of pancreatic tissue is needed to maintain endocrine and exocrine pancreatic function in the normal animal. Feldman & Nelson (2004) recommend against removal of insulinomas from the body (central) part of the pancreas for fear of causing severe, life-threatening pancreatitis, but this has not been the authors’ experience. The blood supply to the pancreas is important and should be reviewed prior to surgery.
The regional lymph nodes should be removed if visible or palpable. Removal of suspected metastases from the liver can be difficult if they appear as multiple small nodules, but a liver biopsy should always be taken for staging purposes. The surgeon should endeavour to remove as much neoplastic tissue as possible, because even if not all the cancer is resectable, debulking can alleviate clinical signs for some months. Survival can be prolonged in dogs that undergo tumour debulking and medical management, compared to dogs treated medically only (Polton et al 2007, Tobin et al 1999).
With surgery alone the MST was 381 days with survival time being unrelated to the location of the tumour within the pancreas (Tobin et al 1999). Caywood et al (1998) reported the following prognosis with surgery: tumours confined to pancreas (stage I) were normoglycaemic for a median of 14 months postoperatively, dogs with metastasis to regional lymph nodes (stage II) or distant metastasis (stage III) were normoglycaemic for a median of about 1 month. Dogs with stage I or II disease had an MST of 18 months, dogs with stage III disease had an MST of <6 months. Dogs treated with partial pancreatectomy will eventually re-present with clinical signs of hypoglycaemia due to metastases. It is advised in these dogs to start medical management at the time because this will greatly increase survival times (Polton et al 2007).
A repeat surgery to debulk tumour and metastases after a relapse of hypoglycaemia may also prolong survival further, especially if in combination with ongoing medical management. Abdominal ultrasound is generally performed to try to assess the feasibility of such a surgery.
Blood glucose levels and surgery
Preoperative, intraoperative (every 30 minutes of surgery time) and postoperative measurements of blood glucose are required, with intravenous supplementation with 2.5–5% dextrose-containing fluids until glucose levels remain stable. A constant rate infusion (CRI) of glucose and dexamethasone can also be used to help prevent severe hypoglycaemia if 5% dextrose supplementation is inadequate. In the authors’ experience, the blood glucose generally starts to climb within a few hours of surgical removal of the insulinoma. Dextrose solutions of 7.5–10% can be utilized, if necessary, through a central (jugular) catheter.
Medical management
For the patient presenting with hypoglycaemic seizures, 1–5 ml 50% dextrose is given slowly intravenously, and the patient is fed as soon as able to eat.
For patients with inoperable insulinomas, or those with evidence of metastases or recurrent clinical signs after successful surgery, medical management can prolong good quality of life for many months. In these patients the goal of medical treatment is to prevent the clinical signs associated with hypoglycaemia. These patients will already have adapted to some degree of hypoglycaemia and, therefore, you do not have to achieve normal levels with treatment but only a level at which the patient is comfortable. However, medical management is no substitute for surgery in the majority of patients. MST for patients on prednisolone only was 74 days in one study (Tobin et al 1999).
Many patients can be kept relatively stable by frequent feeding of diets rich in protein and complex carbohydrates, sometimes up to six meals daily. However, ultimately drug therapy is required.
The first-line drug should be prednisolone. Prednisolone inhibits the action of insulin on peripheral tissue and stimulates glycogenolysis. Initial dose is 1 mg/kg, then reduced to the lowest effective dose.
The second-line drug is diazoxide, which inhibits secretion of insulin by beta cells by blocking calcium mobilization, stimulates gluconeogenesis and glycogenolysis, and inhibits tissue use of glucose, therefore decreasing glucose utilization. Typical dose is 3.3 mg/kg every 8 hours, which can be increased up to 20 mg/kg every 8 hours. Diazoxide should be used in conjunction with prednisolone to maintain blood glucose high enough to prevent signs of neuroglycopenia.
Other agents include:
• Streptozotocin (nitrosourea): selectively toxic to beta cells, it is nephrotoxic to dogs, but safe if administered with aggressive saline diuresis. It can cause diabetes mellitus. There is minimal advantage in using this drug, because dogs treated with surgery alone have similar median disease-free intervals. Streptozotocin may provide a rapid resolution of paraneoplastic peripheral neuropathy (Moore et al 2002).
• Sandostatin (somatostatin analogue): has a wide range of effects and is very effective in humans with insulinomas. In one small study in dogs, four out of five had some response (Lothrop 1989), but it is very expensive.
Glucagonoma
These are rare tumours arising from the alpha cells of the pancreatic islets. The clinical signs are associated with the catabolic effects of glucagon. Diabetes mellitus and superficial necrolytic dermatitis are associated with this tumour (Gross & O’Brien 1989), although not all dogs with this condition have a glucagonoma (Turnwald et al 1989). In patients with clinical signs compatible with glucagonoma serum glucagon levels should be checked.
The treatment of choice for patients with glucagonoma is surgical resection. The number of reported cases is low and all dogs had metastatic disease at the time of diagnosis, making the prognosis poor. CT successfully identified a glucagonoma in one dog; ultrasonography was not found to be helpful. Liver function tests were normal in dogs with glucagonoma, but abnormal in dogs with superficial necrolytic dermatitis secondary to liver disease (Ward & Washabau 2005).
Gastrinoma
These tumours arise from non-beta islet cells of the pancreas. The actual cell of origin is unknown because the adult pancreas does not produce gastrin. The D cells produce gastrin in foetal life but in the adult they secrete somatostatin. Gastrinomas may be associated with multiple endocrine neoplasia (MEN) syndrome.
Clinical signs
Clinical signs include chronic vomiting, anorexia, weight loss, gastroduodenal ulceration and chronic small bowel diarrhoea. It is a rare tumour in dogs but should be considered in any patient with gastroduodenal ulcers that respond well to H2-receptor antagonist therapy but relapse when treatment ceases.
Diagnostic work-up
In patients where a gastrinoma is suspected, a fasting serum gastrin level should be assessed, although levels may be normal in the early stages. Normal = <100 pg/ml in dogs and cats. In dogs with gastrinomas levels are typically in the order of more than three times the upper limit of the normal reference value (Feldman & Nelson 2004). Most (80%) dogs and cats with gastrinoma have gastrointestinal ulceration (Zerbe & Washabau 2000), and endoscopy and ultrasound can demonstrate this. Abdominal ultrasound will not reliably show a pancreatic tumour as they are usually small or microscopic (Roche et al 1982). Scintigraphy with radiolabelled somatostatin analogues often assists in diagnosis (Gibril et al 1996, Schirmer et al 1995) and can also identify which gastrinomas may benefit from octreotide (a long-acting somatostatin analogue which decreases gastrin release) (Ellison et al 1986).
However, surgery is a better way to investigate further if a gastrinoma is suspected based on clinical signs and an elevated fasting serum gastrin level. Surgery allows a definitive diagnosis via pancreatic biopsy, removal of ulcers, and removal of regional lymph node or liver metastases, if possible. Gastrinomas have a predilection for the right lobe and body of the pancreas (Zerbe & Washabau 2000), so removal of the right pancreatic lobe should be performed if no discrete tumour can be found.
Treatment
A combination of drugs have been used, including H2-receptor antagonists such as ranitidine, cimetidine and famotidine; proton pump inhibitors such as omeprazole; diffusion barriers such as sucralfate; synthetic prostaglandins, e.g. misoprostol, and somatostatin analogues, e.g. octreotide.
Prognosis
Gastrinomas are aggressive with metastasis common (regional lymph nodes, liver, spleen and/or mesentery) in 70% of dogs at diagnosis (Altschul et al 1997, Feldman & Nelson 2004, Green & Gartrell 1997). Surgical debulking of tumour reduces gastrin secretion and enhances adjunctive medical management (Zerbe & Washabau 2000). An improved prognosis seems likely with early diagnosis, treatment with surgery and adjunctive medical management. Dogs and cats treated surgically and/or medically survive from 1 week to 2 years, most dying <8 months after diagnosis (Zerbe 1992). Altschul et al (1997) reported that surgery and combination therapy with an H2 blocker, omeprazole, sucralfate and octreotide allowed one canine patient to survive for 14 months. Omeprazole and surgical biopsy resulted in a 2-year survival for another dog (Brooks & Watson 1997). A 26-month disease-free interval with medical management alone (sucralfate, omeprazole and ranitidine) has also been reported (Hughes 2006).
Adrenal gland
Hyperadrenocorticism due to an adrenocortical tumour accounts for ∼15% of naturally occurring hyperadrenocorticism in dogs (Feldman & Nelson 2004, Reusch & Feldman 1991). The ratio of adenoma to carcinoma is approximately 1 : 1 (Feldman & Nelson 2004, Scavelli et al 1986). However, not all adrenal tumours are secretory and may be found during the diagnostic work-up for other problems.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree