The current incidence of bone tumours in dogs is in the order of 7.9/100 000 (Dorn 1976, Dorn et al 1968). Of these, 98% are malignant (Brodey & Riser 1969). Tumours of the bone can be divided into three groups – primary bone tumours, tumours metastatic to bone and tumours that have invaded into bone.
Primary bone tumours
By far the most common tumour of bone is osteosarcoma (OSA). This malignant tumour accounts for approximately 85% of all primary bone tumours (Brodey & Riser 1969). Other tumours include other sarcomas, e.g. chondrosarcoma (CSA), fibrosarcoma (FSA) and haemangiosarcoma (HSA), which account for approximately 5–10% of all bone tumours. Other rare tumours include lymphosarcoma, plasma cell tumours, osteomas, chondromas and other sarcomas, e.g. liposarcoma (Brodey & Riser 1969, Nielsen 1976).
Osteosarcoma
OSA is a malignant mesenchymal tumour of primitive bone cells. These cells produce osteoid (matrix), differentiating them from other sarcomas of bone. A histological diagnosis requires a large enough biopsy so that OSA is not confused with reactive bone, osteomyelitis, CSA, HSA or FSA. OSA is locally aggressive, causing lysis and/or production of bone and soft tissue swelling, and may cause pathological fractures.
OSA arises in both the appendicular and axial skeleton. Large breed dogs with increased height are considered to be at greater risk than small breed dogs (Brodey & Riser 1969, Ru et al 1998). In fact, 90% of all OSA arise in dogs that weigh greater than 20 kg (Brodey & Riser 1969). The majority of canine OSA develop spontaneously but they have also been associated with previous radiation therapy, as a result of fractures, placement of metal implants and as a consequence of bone infarcts (Withrow & Vail 2007).
The appendicular skeleton accounts for approximately 75% of cases, with the metaphyseal region of bone the most common location and tumours arising more frequently in the fore than the hind leg (2 : 1) (Knecht & Priester 1978). The distal radius/ulna is the most common location, accounting for 40% of all OSA. The phrase ‘away from the elbow, towards the knee’ has been coined to describe the most frequently affected sites on the long bones; however, it is important to remember that this is an indication only and OSA are not restricted to these locations. The distal radius and proximal humerus are the most common locations (‘away from the elbow’) (Knecht & Priester 1978). In the hind leg, distribution appears fairly even between distal femur, distal tibia and proximal tibia, with proximal femur less common (Brodey & Riser 1969). It is very unusual for primary OSA to cross a joint surface, or to occur distal to the antebrachiocarpal or tarsocrural joints, or to occur at the proximal radius or distal humerus (Gamblin et al 1995, Liptak et al 2004a).
OSA of the axial skeleton, although less common than their appendicular counterparts, are an important cancer of dogs. Approximately 50% of axial OSA occur in bones of the skull, those affecting the mandible and maxilla being most common. The rib accounts for 25% of axial skeleton OSA. Less frequently seen are those of the cranium, scapula, vertebrae, pelvis and sternum (Heyman et al 1992). The biological behaviour of rib and scapular OSA is more compatible with the appendicular rather than the axial skeleton (Kirpensteijn et al 1994, Matthiesen et al 1992, Pirkey-Ehrhart et al 1995). In the smaller breed dogs (<15 kg) the incidence of OSA, as a percentage of all skeletal neoplasms, is 45% as opposed to 85% seen in the large breeds (Cooley & Waters 1997). Axial and appendicular skeleton are almost equally represented in small breed dogs, with the femur and tibia as the most common appendicular sites (Kistler 1981). Metastatic bone tumours account for about 25% of all bone tumours in small dogs, compared to <5% in large dogs (Cooley & Waters 1997). Finally, extraskeletal OSA is an uncommon and extremely aggressive malignancy (Kuntz et al 1998, Langenbach et al 1998).
Appendicular osteosarcoma
Signalment
• Age: 7.5 years median age with a second peak at 1.5 years (Misdorp & Hart 1979).
• Breed: Large breeds with Rottweilers, Great Danes, St Bernards, Irish Wolfhounds and Greyhounds over-represented (Brodey & Riser 1969, Rosenberger et al 2007). Increasing age (Rosenberger et al 2007), height and weight correlate with increased risk (Ru et al 1998).
• Sex: one large study showed an M:F ratio of 1 : 1 (unpublished data CSU-ACC OS database). Neutered dogs have a higher risk than intact animals (Cooley et al 2002, Ru et al 1998).
Clinical signs
These patients usually present with signs of intermittent lameness that initially is responsive to non-steroidal anti-inflammatory drugs (NSAIDs). In some instances the patient may become acutely lame after running, or the client will notice a hard swelling on the leg. The clinical signs of pulmonary metastatic disease from a primary bone tumour are most commonly inappetence and malaise, and respiratory signs are uncommon. Hypertrophic osteopathy may develop in dogs with pulmonary metastasis.
Physical examination
In many cases the patient will have a hard swelling on the affected leg. Depending on the individual, this may or may not appear to be painful, and in some cases there may be significant soft tissue swelling. The degree of lameness is extremely variable at presentation, from toe-touching lame to non-weight bearing, and seems to have very little correlation with the radiographic signs. Acute lameness with a pathological fracture accounts for <3% of all fractures (Boulay at al 1987). Pain may be elicited on deep bone palpation in early cases, where soft tissue swelling is not obvious.
Diagnostic work-up
Radiography
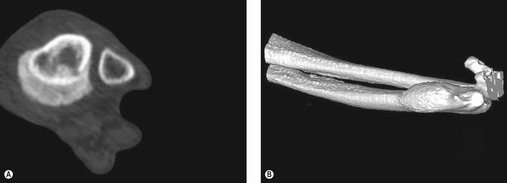
A history of unexplained lameness or the presence of a firm mass warrants radiographic assessment (lateral and craniocaudal views) of the affected bone. Features of an aggressive bony lesion may include one or all of the following (see Figure 21.1A–D): a loss of fine trabecular detail of the metaphyseal bone due to lysis, discontinuity of the cortex, periosteal new bone formation (Codman’s triangle), palisading mineralization perpendicular to the bone shaft (‘sunburst effect’), extension of a mass into the adjacent soft tissues, an indistinct transitional zone between tumour and normal bone, inappropriate areas of sclerosis, or pathological fracture. OSA does not cross the articular cartilage, although tumour may extend into soft tissues and to adjacent bones. Primary lesions usually remain monostotic. CT scans, when available, give a more accurate evaluation of the extent of affected bone.
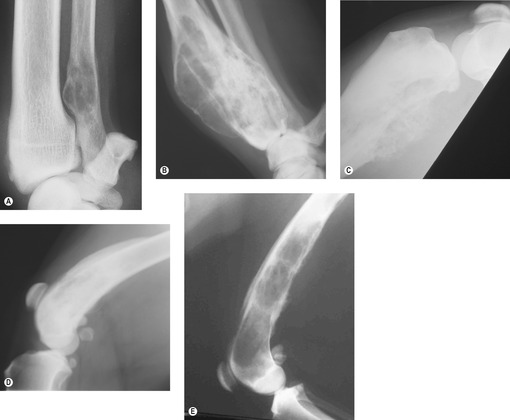 |
| Figure 21.1 (Figure 21.1A, C, E – Courtesy R Straw.) |
The differential diagnosis for a radiographic aggressive bony lesion includes bone tumour (primary or metastatic), infection (Figure 21.1E) (fungal or bacterial), and (rarely) bone cysts.
Bacterial osteomyelitis is suspected on the basis of history (as contamination from surgery or trauma is more common than haematogenous spread). Mycotic osteomyelitis is a major differential diagnosis in countries where fungal diseases are common as they can be monostotic with preference for metaphyseal lesions and spread is haematogenous; however, most mycotic diseases are polyostotic and associated with pulmonary infiltrates and thoracic lymphadenopathy. Travel history and the signalment of the patient may be indicative of a fungal infection.
Clinical staging
Haematology, biochemistry and urinalysis are standard. Routine blood work is often normal; however, there may be an elevation in alkaline phosphatase (ALKP), which may be of value as a prognostic indicator (see below).
Thoracic radiographs (preferably three views) should be taken. Less than 10% of dogs with OSA have visible pulmonary metastases at the time of initial examination; however, if present, the prognosis is poor (Brodey 1965). Bone survey radiographs were more useful in the detection of metastatic lesions (6.4%) than were chest radiographs (4%) in one study of 171 dogs (Straw et al 1989a).
Advanced imaging is useful for the diagnosis of occult metastatic bone disease, and more sensitive detection of pulmonary metastatic disease (CT, MRI, nuclear and CT/PET). CT allows better evaluation of cortical erosion, fractures and internal characteristics such as ossification and calcification (Figure 21.2). It may also be more useful in detecting pulmonary metastatic disease compared to thoracic radiographs. The increased availability of CT does mean that metastatic lesions not seen on plain radiographs are being identified. Whilst this inevitably impacts on our discussions with our clients, all the data currently available on survival times have been derived from patients staged with radiographs. Our ability to see these lesions should not stop us treating patients but may make it easier to explain the importance of follow-up chemotherapy to the client.
In addition to the lung, the most common metastatic site is to bone (Figure 21.3A,B) and care should be taken to evaluate the patient as much as possible for evidence of bony metastases. Other sites include kidneys (Figure 21.3C), spleen, myocardium, lymph nodes (regional and distant), diaphragm, mediastinum, spinal cord, small intestine, gingiva and subcutaneous tissue. A surgical staging system has been devised for human bone sarcomas, and most dogs present with stage IIb disease using this system (Table 21.1).
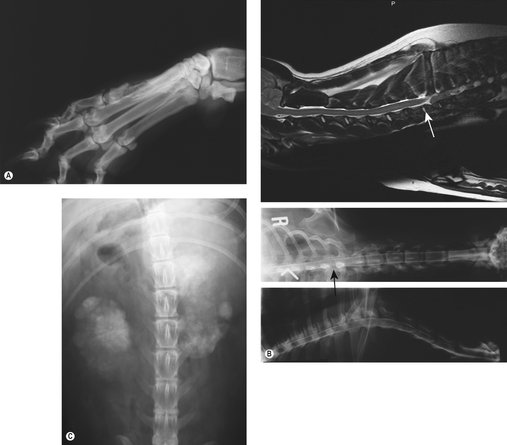 |
| Figure 21.3 (A) Digital bone metastasis with pathological fracture. (B) Spinal metastasis. (C) Renal metastases. |
| *Most dogs with osteosarcoma present with stage IIb. | |
| Stage | Characteristics |
|---|---|
| I | Low grade without evidence of metastasis |
| II | High grade without evidence of metastasis |
| III | Any grade with metastasis |
| A (T1) | Intracompartmental |
| B (T2) | Extracompartmental |
Bone scintigraphy is useful for diagnosing occult metastatic bone lesions. It is indicated for polyostotic bone disease or bone metastasis. Bone scintigraphy cannot differentiate benign from malignant or accurately determine the extent of tumour. It has no value for pulmonary metastasis. A number of studies have looked at the incidence of occult bone metastases using scintigraphy and, depending on the study, between 4 and 28% of patients with OSA may have bony metastases (Berg et al 1990, Hahn et al 1990, Janowski et al 2003, Lamb 1987, Parchman et al 1989). However, the limited accessibility to scintigraphy means that most patients are treated based on plain radiographs. In those patients where the ‘primary’ appears to be located in an atypical location for OSA, scintigraphy, if available, should be considered.
Bone biopsy
Histopathology is required for definitive diagnosis of a bone tumour. Presurgical biopsy techniques include closed (Jamshidi needle), open, or limited open (Michele trephine). The skin incision is made so that the biopsy tract and any potentially seeded tumour cells can be completely removed at the time of definitive surgery. Care is used to avoid major nerves, vessels and joint spaces.
Jamshidi
A 4-inch, 8–11 G bone biopsy needle is used through a small stab incision in the skin. Two to three biopsies of the centre of the lesion (as determined from radiographs) are taken via the same skin incision (only once the cortex is penetrated). The diagnostic accuracy is 82% for specific tumour type and 92% for differentiating tumour from other causes, and complications are uncommon (Powers et al 1988). Post-biopsy radiographs confirm the position of the biopsy tract. Material for culture and cytology should be taken before fixation in formalin. Fluoroscopy is useful for assisting bone biopsy for axial sites.
Open biopsy
Open biopsy guarantees a large biopsy but with increased risk of operative complications (haematoma, infection, dehiscence, tumour seeding, and pathological fracture).
Limited open (Michele)
This has a diagnostic accuracy of 94% but an increased risk of pathological fracture (Wykes et al 1985).
FNA cytology
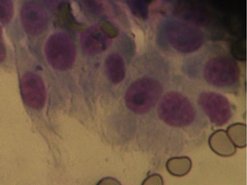
Aspiration of the intramedullary region is performed through areas of cortical destruction (can be ultrasound guided). In dogs, ultrasound-guided needle aspirations of aggressive appendicular bone lesions indicated sarcoma with a sensitivity of 97% and a specificity of 100% in 32 of 36 cases. When a diagnosis of sarcoma was made on cytology (Figure 21.4), ALKP staining indicated OSA, with a sensitivity of 100% (Britt et al 2007).
• Advantages: can be done without general anaesthesia; quicker results; tissue-core biopsy can be performed if FNA is unsuccessful.
• Disadvantages: multiple aspirates are often required for a definitive diagnosis, and excessive blood contamination can interfere with cytological evaluation, particularly in the presence of low cellularity, and cortical bone destruction must be present. Cytology is limited to tumour group and cannot accurately differentiate between the various members of the sarcoma family.
Excisional (postoperative) biopsy
This should always be done to confirm the preoperative incisional biopsy. If a presumptive diagnosis of OSA is made, and limb salvage is not considered an option, amputation can be performed and the tumour then sent for histopathology. A presumptive diagnosis of OSA can be made based on signalment, history, location, physical examination and radiographic features (especially when there is little possibility of fungal or bacterial infection).
Prognostic factors
Medullary OSA is classified according to the predominant mesenchymal component: osteoblastic, chondroblastic, fibroblastic, telangiectatic (vascular) or poorly differentiated. These histological subtypes of OSA in dogs have similar biological behaviour and prognosis. However, the histological grade (based on microscopic features) may be predictive for systemic behaviour (metastasis) (Kirpensteijn et al 2002, Loukopoulos & Robinson 2007). Tumour vascularity may also be prognostic (Coomber et al 1998).
The presence of visible metastatic disease on thoracic radiographs is a negative prognostic indicator (Boston et al 2006), as is the rare finding of a positive lymph node (Hillers et al 2005). Increased serum ALKP (bone, liver or total isoenzymes) has been shown to be a negative prognostic factor (Ehrhart et al 1998, Garzotto et al 2000). Dogs with high bone ALKP before surgery had shorter survival and disease-free intervals. Failure of bone ALKP to decrease after surgery was correlated with shorter survival and disease-free intervals (Ehrhart et al 1998). Dogs with normal pre-treatment total and bone ALKP survived longer than those with increased pre-treatment values (Garzotto et al 2000). In a multi-institutional study of 162 dogs with appendicular OSA treated with amputation alone, dogs younger than 5 years had a worse survival than older dogs (Spodnick et al 1992). Additional studies have related large tumour size (Misdorp & Hart 1979) and humeral location (Bergman et al 1996) to poor outcome.
Clinical stage is prognostic. In a paper of 90 dogs with stage III OSA, the median survival time (MST) was 76 days. Dogs treated palliatively with radiotherapy and chemotherapy lived longer (130 days) than dogs in all other treatment groups. Dogs treated with surgery alone lived 3 days, compared to dogs treated with surgery and chemotherapy (78 days). Dogs with bone metastases had a longer survival time than dogs with soft tissue metastases (Boston et al 2006).
Location of the primary may be influential in determining outcome. For example, in one study looking at OSA of the ulna, 12 cases were examined with a distribution of 1 : 1 metaphyseal to diaphyseal. All were treated with amputation or ulnectomy and adjuvant chemotherapy, and the median survival time was 8.5 months (Straw et al 1991a). OSA distal to the antebrachiocarpal or tarsocrural joints may carry a better prognosis as in one small study (nine cases) the median survival time was 466 days (Gamblin et al 1995).
Scapular OSA has an MST similar to appendicular OSA when treated with surgery and chemotherapy. In many cases a partial scapulectomy can be performed and up to 90% of the scapula can be excised with good to excellent function. Metastatic potential is high and adjuvant chemotherapy (carboplatin) is recommended in these patients (Kirpensteijn et al 1994).
Treatment
The approach to treatment should consider ‘life’ and ‘limb’.
Limb
Amputation is a pain-relieving procedure, and is considered by many to be the best way to relieve the pain caused by the primary tumour. Amputation alone is considered palliative, although the prognosis is better than no treatment (Spodnick et al 1992, Zachos et al 1999). Amputation with or without adjuvant therapy is the standard treatment for appendicular OSA. There are few contraindications to amputation (see Figure 21.5). Concurrent osteoarthritis may progress more rapidly following amputation, although this may be managed medically and most dogs compensate rapidly. Forequarter (including scapula) amputation is required for thoracic limb lesions. Coxofemoral disarticulation is performed for distal pelvic limb lesions and disarticulation combined with en bloc acetabulectomy is used for proximal femoral lesions. Most clients (42/44) were satisfied with the outcome of amputation, and surprised at the rapidity of their dog’s adaptation. No significant association between adaptation with weight, age or thoracic or pelvic limb amputation was found (Kirpensteijn et al 1999).
 |
| Figure 21.5 |
Radiotherapy
This is a palliative treatment for primary and metastatic bone pain. Response and duration of response depend on the size of the lesion, radiation protocol, and whether or not chemotherapy is used in conjunction with radiotherapy. Response (improvement in limb function) occurs in 75–92%, for variable durations (Green et al 2002, Ramirez et al 1999). Reported response duration ranges from 17 to 288 days (median 130 days). In the authors’ experience, dogs with lesions in the proximal humerus do not respond as well as other locations. Typical protocols are 8–9 Gy once weekly for three or four treatments. Radiation should be considered in all patients where surgery is not an option.
Limb-spare
The primary OSA is removed and the limb remains functional by the implantation of another material (e.g. bone autograft, bone allograft, steel prosthesis; see Figure 21.6) or by distraction osteogenesis (Withrow & Vail 2007). Intraoperative radiotherapy has been attempted in a few cases (Liptak et al 2004b). Limb sparing may be used where amputation is declined, or dogs have significant pre-existing orthopaedic or neurological disease. It must be stressed that in the vast majority of cases, amputation is the simplest, quickest, cheapest and best method of treating the primary tumour.
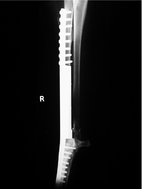 |
| Figure 21.6 (Courtesy R Straw.) |
For a patient to be suitable for a limb-spare procedure, tumour must be clinically and radiographically confined to the limb and involve <50% of the bone (Withrow & Vail 2007). MRI, CT, nuclear scintigraphy and radiographs may be used to estimate the extent of the tumour (Davis et al 2002, Wallack et al 2002). Pathological fracture is a contraindication due to contamination of soft tissues, although this can be reduced by radiotherapy and neoadjuvant chemotherapy. The most suitable sites are the distal radius and ulna (Withrow & Vail 2007).
Surgeons experienced in limb salvage techniques are not always readily available and only experienced surgeons should perform these techniques.
Chemotherapy (Table 21.2)
Cisplatin or carboplatin alone or in combination with doxorubicin have been shown to significantly improve life span. However, irrespective of the protocol employed, the MST remains in the order of 300–365 days with 2-year survival times at 20–25% (Berg et al, 1992 and Berg, Gebhardt, Rand, 1997, Bergman et al 1996, Chun et al 2005, Kent et al 2004, Mauldin et al 1988). Currently, the most widely used protocol is single-agent carboplatin and this is our recommended treatment for middle-aged to older dogs with OSA. Three to four cycles every 3 weeks at 300 mg/m2 have resulted in median survival times of 321 days (Bergman et al 1996). Carboplatin (see Chapter 6) has fewer side effects than cisplatin, does not require diuresis prior to administration and can therefore be given on an outpatient basis. Although it is an expensive drug, the fact that administration is easier means that it is more cost effective than cisplatin.
| See text for references. | |||||
| Drug | Dose | Number of dogs | Median disease-free interval (days) | One- year survival rate | Median survival time (days) |
|---|---|---|---|---|---|
| Carboplatin | 300 mg/m2 IV, 4 cycles at 21-day intervals | 48 | 257 | 35% | 321 |
| Cisplatin | 70 mg/m2 IV, 2 cycles at 21-day intervals | 26 | 177–226 | 38–43% | 262–282 |
| Doxorubicin and carboplatin alternating sequentially | Carboplatin (300 mg/m2) D1, doxorubicin (30 mg/m2) D 21, alternating at 3-week intervals for 3 cycles/drug | 32 | 277 | 48% | 320 |
| Doxorubicin and a matrix metalloproteinase inhibitor (BAY 12-9566) | Doxorubicin 30 mg/m2 every 2 weeks for 5 treatments. | 303 | Not reported | 35% | 8 months |
Combination protocols are generally held to be superior to single-agent protocols (see Chapter 6). However, in the management of OSA there seems to be little benefit to a combination protocol for the majority of patients and when balancing toxicity versus long-term benefit, carboplatin as a single-agent stands up well. Studies alternating doxorubicin and carboplatin or doxorubicin and cisplatin at 3-weekly intervals for three to four cycles/drug did not show any survival benefit over three cycles of carboplatin (median 320 days) (Kent et al 2004, Moore et al 2007). Doxorubicin as a single agent (adjunctive to amputation) every 2 weeks for five cycles resulted in an 8-month MST and a 17% 2-year survival rate in 303 dogs (Moore et al 2007). In younger patients that have a poorer prognosis the authors generally combine doxorubicin and carboplatin but until enough studies are carried out on younger patients the true benefit of this combination remains unknown. Greyhounds show significant myelosuppression with carboplatin that usually results in a reduction of dose to around 250 mg/m2. Treatment delays because of low neutrophil counts are more common with greyhounds than other breeds and, in the authors’ experience, because of regular dose reduction, these patients appear to have an overall poorer prognosis with shorter survival times.
Bisphosphonates
These have been shown to decrease metastatic and primary bone pain, improve quality of life and delay progression of bone lesions. They inhibit bone resorption without inhibiting bone mineralization, allowing stabilization of bone mineral density. As nephrotoxicity is the main side effect, renal parameters should be closely monitored.
Bisphosphonates may be of benefit in those patients not amenable to other forms of therapy. Pamidronate with an NSAID alleviated pain for more than 4 months in 28% of 43 dogs, lasting a median of 231 days (Fan et al 2007).
Currently, the availability of alendronate (an oral bisphosphonate) makes dosing easier. This drug appears to be well tolerated.
Expected prognosis based on treatment
• No treatment: marked pain due to extensive destruction of bone and surrounding soft tissue. Euthanasia usually elected soon after diagnosis due to pain.
• Analgesics only: use of bisphosphonates, NSAIDs, opioids and other analgesics for medical management of the primary tumour still rarely increase survival beyond 3 months.
• Amputation alone: MST is 119–175 days with a 12-month survival rate of 11–21% and 24-month survival rate of 0–4%. Death is due to metastatic disease, usually to the lungs (Mauldin et al 1988, Spodnick et al 1992, Straw et al 1991b, Thompson & Fugent 1992).
• Amputation or limb-sparing and adjuvant chemotherapy: MST is 262–540 days with a 12-month survival rate of 33–69%. This depends on the chemotherapy protocol, stage of disease, location and signalment (Berg et al, 1992 and Berg, Gebhardt, Rand, 1997, Bergman et al 1996, Chun et al, 2000 and Chun et al, 2005, Kent et al 2004, Mauldin et al 1988, Moore et al 2007, Straw et al 1991b, Thompson & Fugent 1992). Most protocols include small numbers of dogs where all factors concerning each individual patient are considered as equal. However, the most important point is that chemotherapy increases the MST for patients with OSA and should be recommended. The other interesting point is that, irrespective of how we combine drugs, the MST still remains in the order of 10–12 months. Interestingly, the MST for dogs that develop an infected limb-spare is increased to 2 years (Devitt et al 1996). Although infection increased morbidity, it has generated an interest in how to stimulate the immune system in conjunction with chemotherapy to improve survival times in these patients.
• Radiation therapy alone: response (improvement in limb function) occurs in 75–92%, for variable durations. Response durations of 17–288 days (median 130 days) have been reported (Green et al 2002, Mueller et al 2005, Ramirez et al 1999).
• Radioactive samarium: this is a radioactive isotope, which is injected intravenously. In 32 dogs with appendicular tumours, 63% had an improvement in the severity of lameness 2 weeks after administration of the first dose, 25% had no change in the severity of lameness, and 12% had a worsening. Median survival time was 93 days, with 9.4% alive after 1 year (Barnard et al 2007).
Axial osteosarcoma
Axial (cranium, mandible, maxilla, nasal cavity, spine, rib and pelvis) bone tumours may present with other signs. For example, skull or nasal cavity bone tumours may present with a mass on the skull, seizures, sneezing, exophthalmos, pain on opening the mouth, facial deformity, or a nasal discharge. Tumours arising from the rib(s) may present with a palpable rib mass, dyspnoea, pain, or rarely pleural effusion. Vertebral bone tumours may present with spinal pain and/or neurological deficits. Bone tumours of the pelvis cause a pelvic outflow obstruction (tenesmus, stranguria) more commonly than lameness.
The biological behaviour for axial OSA appears to be similar to appendicular OSA (aggressive), with the exception of the mandible and possibly the rest of the calvarium (Hammer et al 1995). Pulmonary metastasis was seen in 11% of canine axial OSA (Heyman et al 1992). The metastatic rate at diagnosis is 27% for rib OSA and 17% for skull OSA (Feeney et al 1982).
In general, the approach to treatment of the axial skeleton is different from that for the appendicular skeleton, and tumour-related death in these patients is usually a consequence of local tumour recurrence (80%) rather than metastatic disease (7%) (Heyman et al 1992). Tumours of the scapula and rib should be treated as appendicular OSA with surgical excision and carboplatin chemotherapy. One of the major problems with OSA of the axial skeleton is the surgeon’s ability to completely excise the tumour and that directly translates to location and size of tumour on presentation. Primary OSA of the axial skeleton appears distributed at 27% mandibular, 22% maxillary, 15% vertebral, 14% cranial, 10% ribs, 9% nasal cavity/paranasal sinuses and 6% pelvic (Heyman et al 1992).
A number of reports in the literature give varying overall survival times for patients with axial OSA. The major prognostic indicator appears to be size, with smaller dogs having a much better prognosis than large breed dogs (Dickerson et al 2001).
Mandible
As expected, OSA of the mandible carries the best prognosis of all axial OSA locations with tumour invading local bone but pushing rather than invading adjacent soft tissues. In patients where surgical excision margins have been achieved, further therapy is rarely indicated as 12-month survival rates with partial mandibulectomy alone are about 70% (Straw et al 1996). There is a potential benefit of adjuvant chemotherapy for the 30% of cases that do not survive 12 months with resection of mandibular OSA with clean margins. This subset tends to fail due to metastatic disease within the first 3 months.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree