In this section a general discussion of the STS family follows. The section is divided into canine and feline sarcomas to facilitate emphasizing some of the differences encountered in the two species, but the overall characteristics of STS will be described under canine sarcomas.
CANINE SARCOMAS
Introduction
The name ‘sarcoma’ is derived from the Greek word sarkoma, meaning ‘fleshy growth’, and virtually all members of the ‘family’ are derived embryologically from mesoderm. It is the primitive mesoderm that gives rise to the connective tissues of the body and STS include those tumours that arise from extraskeletal connective tissue whose function it is to connect, support and surround other discrete anatomical structures. Tumours of the Schwann cells are also included in this group, even though they arise from the neural tube of the primitive ectoderm.
STS are characterized by the possession of a pseudocapsule that is composed of compressed tumour cells providing no ‘capsular’ barrier to the local spread of tumour cells. Morphologically they are distinct, but because their biological behaviour is similar, the fine distinctions between these tumours are often no longer reported and they are described as STS without reference to the cell of origin. Tumours considered STS are listed in Table 20.1; other sarcomas that are found within the skin and subcutaneous tissues, including haemangiosarcomas (HSA) and histiocytic sarcomas (HS), are not considered as STS because of their more aggressive biological behaviour and are discussed separately. STS as a family typically have a low–moderate metastatic potential and predominantly spread haematogenously.
| + low metastatic potential; ++ moderate metastatic potential; +++ high metastatic potential. | ||
| Tissue of origin | Malignancy | Metastatic potential |
|---|---|---|
| Fibrous | Fibrosarcoma | +/++ |
| Adipose | Liposarcoma | +/++ |
| Schwann cell | Schwannoma/nerve sheath tumour | +/++ |
| Pericyte | Haemangiopericytoma | + |
| Fibrous + giant cells | Malignant fibrous histiocytoma (does not stain positive for histiocytic markers) | +/++ |
| Skeletal muscle | Rhabdomyosarcoma | ++ |
| Lymphatics | Lymphangiosarcoma | ++ |
| Smooth muscle | Leiomyosarcomas | +/++ |
| Synovium | Synovial cell sarcoma | +/+++ |
| Unknown | Anaplastic (poorly differentiated) | +++ |
Incidence and risk factors
STS are relatively common tumours with a generally unknown aetiology. Sarcomas may develop in association with radiation, trauma, foreign bodies, orthopaedic implants, Spirocerca lupi infestation, and in cats with feline sarcoma virus, vaccines/other subcutaneous injections, and trauma (e.g. intraocular sarcoma) (MacEwen et al 2001). STS accounts for approximately 15% of all canine tumours (compared to 0.7% in humans) and are more common in medium to large breed dogs, of middle to older age (10 years). However, any age or breed of dog or cat can be affected. There is no sex predilection.
Clinical signs
STS will often appear as a single, soft to firm, rounded, non-painful, fixed or mobile subcutaneous mass in any site, including head and neck, but most often over the trunk or extremities. The distribution of STS has been reported as 40% trunk, 25% proximal to the knee, 12% proximal to the elbow, 7% distal to the elbow and 9% distal to the knee. STS demonstrate a variable growth rate, although most are commonly slow growing, with symptoms related to the location of the mass and the degree of invasion.
Diagnostic work-up
Initially, the clinician should perform a complete physical examination of the patient. The mass should be palpated to give an idea of site, size and fixation to underlying or adjacent structures. The regional lymph nodes (RLN) are also palpated for enlargement and fixation to underlying tissues (remember most STS will not metastasize via regional lymph nodes).
Fine needle aspirate (FNA) cytology of the mass should be performed. This is primarily to exclude other differential diagnoses such as mast cell tumour, infection, inflammation, lipoma, etc. In general, STS do not exfoliate well on FNA, and it is the more aggressive tumours that are likely to be positive for neoplastic cells on cytology. Often it is the absence of cells obtained from an aspirate that increases the suspicion of an STS and should then prompt a biopsy (see Chapter 5). In one study of 40 dogs with STS, FNA cytology obtained an incorrect diagnosis in 15%, a further 23% were non-diagnostic and only 62% were correctly diagnosed (Baker-Gabb et al 2003).
A definitive preoperative diagnosis and histopathological grade are obtained from tissue biopsy (punch, needle core, incisional). Tumour grade is important for predicting prognosis and for treatment planning. The biopsy tract should be positioned so that it is excised at curative-intent surgery, or included in the radiation field (without increasing the size of the radiation field). Excisional biopsies/marginal resections are not recommended. Subsequently, more aggressive surgery is required to achieve complete histological margins, with greater morbidity and expense than if incisional (wedge or core) biopsies were originally performed.
The survival time in dogs with STS is also shortened with multiple attempts at resection (Posterino et al 1988). The chance of a surgical cure is greatest with the first surgery (Banks et al, 2003 and Banks et al, 2004, Graves et al 1988, Kuntz et al 1997, Liptak et al 2007, MacEwen et al 2001, Posterino et al 1988). As there is also a tendency for selection of more malignant cell populations with repeat surgeries, low-grade tumours can, with time, become more malignant and likely to metastasize.
The metastatic potential is reflected in the grade of STS; less than 10% grade I, 20% grade II and 50% grade III undergo metastasis (Kuntz et al 1997). High-grade (III) tumours are characterized by a higher mitotic rate, greater percentage necrosis and increased metastasis (Bostock & Dye 1980, Graves et al 1988, Kuntz et al 1997). Locally, these tumours tend to grow more rapidly, be more invasive and show higher rates of local recurrence (Bostock & Dye 1980, Graves et al 1988, Kuntz et al 1997) (see Table 20.2 for the staging system commonly used for canine STS). It should be noted that the significant improvement in the management of primary STS has resulted in a greater proportion of patients living many years after surgery, with the consequence that late metastases are seen.
| *Mitotic index: number of mitotic figures/10 high-powered fields (40×) | |||
| Reproduced with permission from Kuntz et al (1997). | |||
| Grade | Degree of differentiation | Mitotic index* | Necrosis |
|---|---|---|---|
| Low (1) | Normal appearance | <10 | None |
| Intermediate (2) | Histologically identifiable | 10–19 | <50% |
| High (3) | Undifferentiated | >19 | >50% |
Routine haematology, biochemistry and urinalysis are usually normal for dogs with STS that adhere to the traditional classification.
The most important tool for staging of metastatic disease is either thoracic radiographs or thoracic CT. Palpable RLN metastasis occurred in 0% in two studies (Banks et al, 2003 and Banks et al, 2004), and in 6% in another study (Kuntz et al 1997); however, FNA or biopsy of RLN should be performed in dogs with clinically abnormal lymph nodes (Liptak et al 2007).
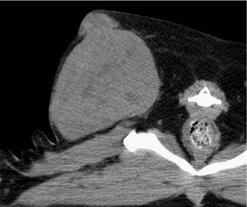
Abdominal ultrasonography or other advanced imaging may also be employed to assist in further staging in selected cases. The surgeon and/or radiation oncologist may also benefit from further imaging of the local tumour. Routine radiography, ultrasonography, CT, MRI, nuclear scintigraphy or angiography may be useful for planning either the surgical approach or radiation therapy, especially for large fixed STS or STS in close proximity to vital structures (Figure 20.1). CT three-dimensional (3-D) images are especially useful for surgical planning, particularly if an aggressive resection with curative intent is planned (Figure 20.2).
 |
| Figure 20.1 (Courtesy R Straw.) |
Treatment
After staging is complete, treatment options are considered. This is because the presence of metastatic disease changes the goal of treatment to palliation rather than potential cure. In the absence of metastatic disease, the treatment of choice is surgical excision of the tumour with wide margins and one fascial plane deep. The most important surgical consideration is not to be misled by the pseudocapsule into shelling these tumours out. These peripheral cells can be more aggressive and may be responsible for the extensive invasion into adjacent normal tissues characteristic of STS. If these potentially more aggressive peripheral or deeply invasive cells are left behind, tumour regrowth is expected. Incomplete resection of canine STS has been reported to result in a probability of local recurrence of 60% within the first 12 months (Bostock & Dye 1980). In another study, 28% of dogs with incomplete STS resection had local recurrence, and were over 10 times more likely to have local recurrence than dogs with complete excision (Kuntz et al 1997).
Surgical resection with wide margins
It is generally well accepted that surgical resection with wide margins provides the best chance for long-term control, survival and cure for patients with STS. ‘Wide margins’ refers to the minimum recommended surgical margins, i.e. 3 cm of grossly normal tissue lateral to the tumour and at least one fascial layer deep to the tumour (Baker-Gabb et al 2003, Banks et al, 2003 and Banks et al, 2004, Dernell et al 1998, Kuntz et al 1997, Posterino et al 1988). Surgical resection with wide, histologically complete, quantitatively evaluated surgical margins resulted in 90–100% local disease control rate, and a 90–93% 1-year disease-free survival in two studies (Evans 1987, McChesney et al 1989).
Another study reported that canine STS removed with attempted wide margins, regardless of completeness of excision, had an 85% rate of local tumour control with a median time to recurrence of 368 days (Kuntz et al 1997). In a third study, 79% of canine STS resected with wide, complete, histological margins were controlled for 2 years (Posterino et al 1988). In 56 dogs with liposarcomas, surgical dose was the only factor significantly associated with survival time (Baez et al 2004). Resection of liposarcomas with wide margins achieved a median survival time (MST) of 1188 days, compared to 649 days for marginal excision and 183 days for incisional biopsy.
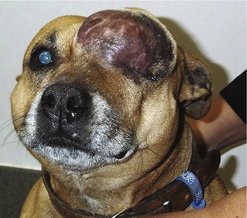
As a chance for cure rests with an adequate surgical dose, referral to a specialist facility should be considered for difficult cases, especially where advanced imaging facilities are required. Achieving wide clean margins can be a surgical challenge, but as it affords the best prognosis, it is a worthy undertaking. As mentioned above, associated biopsy tracts and any areas of fixation (e.g. bone, fascia) should be removed en bloc with the tumour (Figure 20.3).
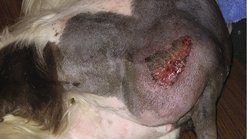 |
| Figure 20.3 |
Surgeons should familiarize themselves with cutaneous reconstructive techniques. Local pedicle flaps (e.g. advancement, rotation, transposition) and axial pattern flaps of the head and neck (caudal auricular, omocervical, thoracodorsal) may assist in closing large skin deficits of the head and neck. Detailed, valuable descriptions of skin flaps are found in Pavletic’s Atlas of Small Animal Reconstructive Surgery (1999).
It should also be remembered that for STS of the distal extremities amputation is an option for achieving wide surgical margins. In most situations client preference is to save the limb if possible, but for large tumours, those that are circumferential, where radiotherapy may not be accessible, or where financial considerations impact on future treatment, amputation is a very cost effective and acceptable procedure. It should also be considered in patients that have failed cytoreductive surgery and/or radiotherapy.
Marginal resection and adjuvant radiation therapy
If the tumour cannot be excised with wide margins, marginal resection followed by adjuvant radiotherapy is recommended to increase disease-free interval and survival time. Generally, the use of radiation is restricted to the extremities and head and neck where the achievement of surgical margins is more complicated and recurrence more difficult to manage. Local control is better with higher cumulative doses, but the optimal treatment protocol has not yet been determined (Forrest et al 2000, McKnight et al 2000).
Marginal resection followed by fine fractionated megavoltage radiotherapy has been reported in two studies to result in a 16% local recurrence rate with a median time to recurrence of 700 days to more than 798 days (Forrest et al 2000, McKnight et al 2000). Radiotherapy can be utilized prior to surgery in an attempt to decrease tumour volume to allow a wide surgical excision. The use of radiotherapy should ideally involve a team approach with the surgeon and the radiotherapist from the outset, rather than employing radiotherapy as a rescue procedure. Consideration must be given to potential complications of radiation to the structures of the head and neck (e.g. eyes, spinal cord, brain), which may lead to oronasal fistula, bone necrosis, etc. (Chapter 7).
Radiation therapy alone
Radiotherapy alone is generally considered palliative. It may achieve local control in 48–67% of tumours in the first 12 months, with higher cumulative radiation doses resulting in better control rates. However, this rate of local STS control is reduced to 20–33% at 2 years (McChesney et al 1989).
Chemotherapy
Situations in which chemotherapy should be considered include metastatic disease and dogs with high-grade STS (MacEwen et al 2001). However, the efficacy of chemotherapy in the management of sarcomas of the head and neck is not supported by data from previous trials. One report in the literature did not show an increase in survival time (Selting et al 2000) and most showed partial responses of a short duration (Henry et al 1999, Ogilvie et al, 1989 and Ogilvie et al, 1991, Rassnick et al 2000).
Metastatic disease
The presence of metastatic disease changes treatment goals considerably. Management should be with intent to palliate rather than to cure. Therefore aggressive surgery and/or radiation therapy is generally not warranted. The role of pulmonary metastectomy for STS is unknown.
Prognostic factors for local tumour recurrence
Prognostic factors for local tumour recurrence include tumour location, size, completeness of excision and grade. STS in superficial sites or the extremities have a better prognosis than those which are deep, truncal, invasive or close to the spinal cord. In these situations, there is a diminished ability to achieve complete surgical resection.
Incomplete resection is over 10 times more likely to result in local disease recurrence (Kuntz et al 1997). Thickness of deep margin and tumour grade (low and intermediate more favourable) are prognostic factors for local recurrence (Banks et al, 2003 and Banks et al, 2004, Bostock & Dye 1980, Kuntz et al 1997, Posterino et al 1988). Tumours that are freely movable may have a more favourable prognosis than those fixed to underlying tissues (Banks et al 2004). Increased intratumoral microvessel density (IMD), a measure of tumour angiogenesis, is associated with higher histological grade, necrosis scores and mitotic scores for canine STS (Luong et al 2006).
Prognostic factors for survival
Uncontrolled local disease is most often the cause of tumour-related death from STS. Mitotic rate and percentage tumour necrosis were the variables significantly associated with survival time on multivariate analysis in one article (Kuntz et al 1997). Mitotic rate (among other factors) was a significant prognostic factor for survival (univariate analysis) in another article. However, the only significant prognostic factor for survival on multivariate analysis was an increased argyrophilic nucleolar organizing regions (AgNOR) score, an indicator of nuclear activity and cellular proliferation (Ettinger et al 2006).
Other prognostic factors for overall survival include tumour size, completeness of excision, degree of differentiation, histological grade and local tumour control (Kuntz et al 1997). Increased IMD was not prognostic for survival (Luong et al 2006). Dogs with non-oral STS treated with wide surgical resection had an MST of 1416 days. Similar dogs treated with marginal surgical resection combined with adjuvant megavoltage radiation showed a 2270-day MST (Forrest et al 2000, Kuntz et al 1997).
Sarcomas that are typically members of the STS family but with distinguishing features
Myxosarcomas
These rare STS deserve a special mention. What distinguishes these tumours from other members of the STS family is the production of large amounts of mucinous material. This means that on examination they may appear fluid, almost cyst-like, and an FNA can be very misleading, yielding mucinous fluid that can be interpreted as a cyst. Biologically, they behave as any ‘classical’ STS, i.e. that is locally invasive, but with a low–moderate metastatic rate. Locally, however, they can be extremely challenging because the mucinous secretion makes it extremely difficult for the surgeon to determine tumour boundaries. Incomplete resection of a myxosarcoma should be treated as for any other STS with adjuvant radiation.
Lymphangiosarcomas
Again, these are rare tumours that warrant special mention. As with myxosarcomas, diagnosis may be difficult in the initial phases as the patient may present with diffuse swelling or ‘weeping’ skin. Lymphoedema is characteristic of these tumours and surgical control can be frustrating because of the diffuse nature of the tumour itself as the lymphoedema spreads. They are seen typically on the distal extremities and in cats aggressive lymphangiosarcomas have been seen on the ventral abdomen. Advanced imaging (MRI/contrast-enhanced CT) may be beneficial in delineating the extent of the tumours, but prognosis is dependent on adequate excision or combination treatment with radiation.
Other sarcomas
Haemangiosarcomas (HSA)
HSA of the skin and subcutaneous tissue also deserve a special mention. Small primary cutaneous tumours may be either primaries or secondaries, so a full diagnostic evaluation is indicated. Cutaneous metastases from visceral HSA carry a poor prognosis. Small primary cutaneous HSA that can be completely excised with margins have a good to fair prognosis as the metastatic rate is low. Large, deeply infiltrated HSA are aggressive and should be managed like STS with excision with wide margins, and if margins are not achieved, adjuvant radiotherapy. The risk of developing distant metastases is high so staging prior to surgery is indicated. These tumours are usually primaries and the major metastatic site is the lung. In these patients the authors recommend adjuvant chemotherapy (doxorubicin).
Multiple cutaneous HSA is a further manifestation that is rarely encountered but can be frustrating to deal with. Typically, these tumours are small and initially surgical excision is the treatment of choice; however, this may become impossible due to the large numbers of tumours that may develop. Doxorubicin may be beneficial; however, reports on this are lacking. Intralesional interferon alpha (IFN-α) is effective in human paediatric patients with large inoperable haemangiomas and may have application for veterinary patients.
Sarcomas affecting the joint
Synovial cell sarcomas
Synovial cell sarcomas are malignant tumours that arise from the mesenchymal cells within the tenosynovial tissues of joints, bursas and tendon sheaths. The most commonly affected joint is the stifle, followed by elbow, shoulder, carpal, tarsal and hip joints. The mean age at presentation is 6–8 years, with Flat-coat Retrievers (FCR) and males over-represented. Previous reports have shown that up to a third of dogs have metastasis to regional lymph nodes and lungs at the time of diagnosis, and ultimately up to 50% of dogs will develop metastasis.
Previously, synovial cell sarcoma was the most commonly diagnosed neoplasm of the joint, but other sarcomas, particularly histiocytic sarcomas, are now more prevalent. In many cases immunohistochemistry is required to distinguish these tumours. Knowing the predisposition of FCR to develop sarcomas, particularly HS, the above over-representation of this breed for developing synovial cell sarcomas may require modification in the light of current knowledge of Histiocytic Sarcomas (HS) (see below).
Clinical signs and diagnostic work-up
Typical presentation is lameness. Physical examination of the affected joint will elicit pain and effusion. Palpation of regional lymph nodes is required and an FNA if enlarged.
Radiographs of the joint will often show soft tissue opacity adjacent to the joint; mineralization is rare. Bone involvement may be seen in some cases that can be either punctate lysis of bone due to invasion by tumour cells or smooth due to pressure necrosis from tumour. Thoracic radiographs or CT are required to check for thoracic metastasis.
Definitive diagnosis requires biopsy, as synovial cell fluid is rarely diagnostic and usually is consistent with low-grade chronic inflammation. Techniques for obtaining a biopsy include open wedge or Jamshidi needle.
Histologically, synovial cell sarcomas have two distinct populations of cells, epithelioid and spindle. However, in many cases, this morphological distinction is not sufficient to differentiate synovial cell from other sarcomas. Immunohistochemistry is recommended, with synovial cell sarcomas staining positive with cytokeratin antibody AE1/AE3, HS with CD18 antibody and malignant fibrous histiocytomas with smooth muscle actin antibody.
Treatment
The treatment of choice for sarcomas of the joint is high amputation of the affected limb. The role of radiation is unknown, but may be conceived as palliative treatment for patients in pain in which amputation is not an option, either because of other orthopaedic problems or client preference.
The role of chemotherapy is also unknown, but in humans chemotherapy does not improve overall survival times. Should chemotherapy be considered in a veterinary patient the drug of choice is doxorubicin. The patients most likely to receive benefit from chemotherapy would be those with high-grade tumours.
Prognosis
Prognostic factors include:
• clinical stage
• histological grade
• treatment.
Clinical stage
• T1: well defined with no invasion into local structures
• T2: involving soft tissues
• T3: invading bone and joint.
Local disease, irrespective of stage, has not been shown to be prognostic; however, metastatic disease (either regional or distant) warrants a poor prognosis with an MST less than 6 months.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree