Perhaps the most difficult of all the tumours we deal with in veterinary medicine is the mast cell tumour (MCT). Because it is a common tumour, every veterinary surgeon at some point in their career will be pulling their hair out trying to decide on the correct course of action for an individual patient. This is because not only can MCT present with many variations in appearance (the great pretender), the management of the tumour itself and its biological behaviour can be difficult to predict.
CANINE MAST CELL TUMOURS
Incidence
MCT are the most common cutaneous tumours seen in the dog (16–21%) (Bostock 1986, Rothwell et al 1987). They affect mostly older dogs with a mean age of 9 years, but the age range is considerable (3 weeks to 19 years) (Davis et al 1992, Patnaik et al 1984). Breeds with bulldog ancestry are over-represented – Boxers, Bull Mastiffs, Boston Terriers. Other breeds with a predisposition to develop MCT include Labrador Retrievers, Golden Retrievers, Shar Peis, Weimaraners, Schnauzers and Beagles. In the author’s (TB’s) experience in Australia, Staffordshire Bull Terriers are also at high risk and often develop high-grade tumours. No sex predilection has been documented.
Aetiology
Aetiology is unknown, but genetic alterations in expression of c-kit (a stem cell factor membrane receptor, capable of triggering cell growth and normally expressed on haemopoietic and mast cells) have been shown in some canine MCT (Dank et al 2002, Downing et al 2002, Jones et al 2004, Reguera et al 2002, Riva et al 2005, Zemke et al 2002). It has also been proven that canine MCT cells express c-kit (London et al 1996), and dysregulated expression of c-kit may contribute to MCT development (Turin et al 2006).
Clinical signs
In the majority of cases clinical signs are associated with the primary tumour; rarely, systemic signs will occur as the initial complaint.
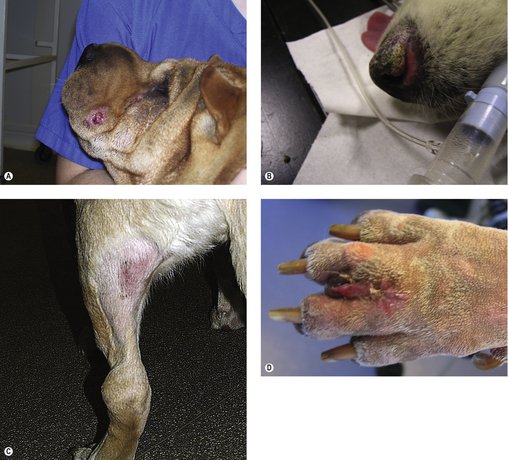
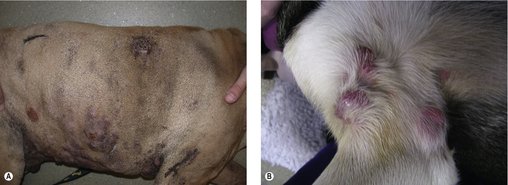
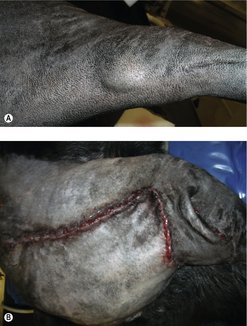
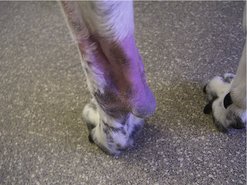
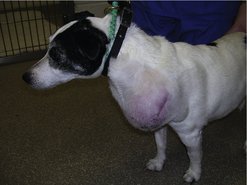
The clinical appearance of MCT is extremely varied, so any skin lump could be an MCT, regardless of its appearance (Figure 19.1). Fine needle aspirate (FNA) cytology and/or histopathology are needed to confirm the presence of an MCT. The majority of dogs present with solitary lesions, but 11–14% have multiple tumours (Figure 19.2) (Mullins et al 2006, Tams & Macy 1981, Van Pelt et al 1986).
A long, stable history is more consistent with lower-grade MCT. High-grade MCT tend to be rapidly growing and the surrounding tissues may be inflamed and oedematous; occasionally small satellite nodules are present.
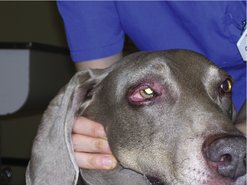
The dermis and subcutaneous tissues are frequent MCT sites, with up to 50–60% of cases on the trunk, 25% on the limbs and less frequently on the head and neck. Other unusual locations include conjunctiva (Figure 19.3), salivary gland, nasopharynx, larynx and oral cavity (Bostock 1973, Iwata et al 2000, Rothwell et al 1987).
 |
| Figure 19.3 |
Darier’s sign results from the mechanical manipulation of MCT, causing degranulation with resultant erythema and wheal formation in surrounding tissues.
Gastrointestinal problems, e.g. vomiting (possibly with blood), anorexia, melaena and abdominal pain, may result from MCT degranulation.
Pleural and peritoneal effusions in dogs with disseminated mastocytosis may contain neoplastic mast cells.
Diagnostic work-up
Clinical examination
The clinician should be aware that tumour location affects tumour behaviour and prognosis (see ‘Prognostic factors’ below). A thorough search of the animal for multiple cutaneous masses should be performed and any new lump found should undergo FNA cytology. If multiple cutaneous MCT are found, the approach to treatment is often different from that for a single MCT.
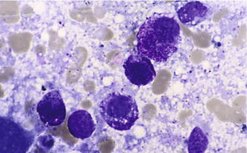
Initial diagnosis is via FNA cytology
The majority of MCT can be identified on FNA. They appear as small-to-medium round cells, usually abundant and with uniform cytoplasmic granules that stain purple-red (metachromatically) (Figure 19.4). However, grade cannot be determined by cytology and it is important to remember that not all MCT stain with Romanovsky-type stains, giving them an epithelial or macrophage-like appearance on the slide. In such cases, histology and possibly immunohistochemistry are required for diagnosis. In some cases FNA of an MCT will only yield blood.
Evaluation of the primary tumour
Prior to surgery it is important to assess the extent of the local tumour. In some cases this can be very difficult when the tumour is fluctuant and does not appear to have defined margins. It can also be difficult in those tumours that constantly wax and wane. Whenever an MCT undergoes a local inflammatory response resulting in a transient increase in size, it must be assumed that tumour cells extend into the affected area. Therefore, the presurgical use of steroids to reduce tumour size results in underestimation of the size of the tumour and potentially incomplete resection. Shrinkage of a tumour before surgery with radiotherapy, however, is completely different in that the edges have been truly sterilized.
The extent of the tumour can be estimated by physical examination, radiographs, ultrasound or computed tomography (CT) scan. Interestingly, in one study, dogs with cutaneous MCT had the extent of the margin upgraded in 19% of cases using ultrasound and 65% of cases using CT (Hahn et al 1990). This is important for planning surgery and radiotherapy. What additional tests are required to establish the extent of the tumour depends on its location and whether a wide excision will be simple or difficult.
Staging
What is the importance of staging?
The more information that is obtained, the more accurate the predictions as to prognosis. In terms of treatment this means that the most appropriate course of treatment can be determined. It is also important to understand the relevance of normal responses in the body that may account for the presence of mast cells. Interestingly, MCT do not metastasize or originate in the lungs.
Additional presurgical diagnostics include the following.
• Evaluation of the regional lymph node (RLN): If the node can be palpated, FNA should be undertaken (even if normal in size). When interpreting a cytological report in which a small number of mast cells are found it is important to remember that mast cells are normal components of lymph nodes. If the lymph node is potentially positive for regional spread, excisional biopsy at the time of surgery is indicated. It is the morphological features of the mast cells in addition to their distribution throughout the node that define early metastasis. Obviously, if the node is significantly enlarged and full of mast cells, cytology is sufficient for diagnosing metastatic MCT.
• Radiographs are of less value in patients with MCT than any other neoplastic process and can usually be eliminated from the work-up of a young patient. In older dogs it is advisable to take thoracic radiographs to ensure that there are no other problems, but it is important to remember that the lung is not a metastatic site for MCT. It is also important to understand the drainage pattern of the lymph nodes to ensure that the sentinel node is checked. Occasionally, intrathoracic nodes will be enlarged in patients with MCT of the forelimbs, and sub-iliac lymph nodes in patients with hindlimb involvement; the sentinel node for aural MCT is the superficial cervical (or pre-scapular) node.
• Abdominal ultrasound to evaluate the liver and spleen is a more valuable diagnostic tool than abdominal radiographs. Abnormalities in these organs (usually hepatosplenomegaly) and a ‘Swiss cheese’ appearance should be evaluated by FNA. The major non-neoplastic differential diagnosis for this ‘punched-out’ appearance in the spleen is splenitis. Disseminated MCT in either the liver or spleen can be diagnosed on FNA; however, a small number of mast cells should be viewed cautiously as these cells can normally be present in these locations, especially if they are morphologically normal.
• Buffy coat analysis is currently rarely of value in the work-up of the patient with MCT unless the patient is physically unwell, e.g. vomiting, diarrhoea, etc., or has very aggressive MCT affecting major organ systems.
• For the same reason as in buffy coat analysis, bone marrow aspirates are rarely performed. A bone marrow aspirate is more accurate than buffy coat analysis. Patients with a positive bone marrow have a poor prognosis.
• Incisional biopsies can be carried out to establish grade prior to surgery.
What should be the extent of staging prior to surgery?
One approach is this:
• If the tumour is excisable with wide margins, and there are no negative prognostic factors, further staging is not needed, and regular postoperative rechecks for local recurrence and palpable RLN enlargement should be sufficient. Further staging could then be performed post-operatively for grade II or III mast cell tumours.
• However, if the MCT is not amenable to wide excision (e.g. distal extremity), or if negative prognostic factors exist in the history or physical examination (see above), ancillary diagnostic tests to stage disease are performed prior to definitive treatment to better advise clients of probable outcomes and to help plan the type and extent of definitive therapy.
Prognostic factors
Known prognostic indicators are as follows.
Histological grade
This is strongly predictive of outcome (Thamm et al 2006). In spite of some ‘greyness’ in defining histological grade, it is highly predictive for overall survival time, providing appropriate therapy is given from the beginning. The vast majority of dogs with well-differentiated tumours (80–90%) and 60–75% of moderately differentiated tumours experience long-term survival following surgical excision ± radiotherapy. Dogs with undifferentiated (high-grade) tumours treated surgically frequently die of their disease within 12 months due to local recurrence or metastasis.
Individual histological criteria such as invasiveness and the number of mitotic figures were prognostic in one study. These parameters may be more reliable than the Patnaik grading system, which is influenced by more subjective observations such as tumour heterogeneity, cellular differentiation and nuclear morphology (Preziosi et al 2007).
Clinical stage
In combination with histological grade, stage is predictive of long-term prognosis (see Table 19.1). Patients with stage 0-I disease confined to the skin without local lymph node involvement or distant metastasis have a better prognosis than patients with stage III–IV disease. At least one study has suggested that there is no difference in outcome between patients with a single MCT and those with multiple cutaneous MCT (Mullins et al 2006, Simoes et al 1994, Thamm et al 2006).
| * The WHO staging system lists multiple dermal MCT as clinical stage III disease. However, multiple MCT confined to the dermis have not been shown to have a worse prognosis, and are not analogous to a large, infiltrating MCT. | |
| Stage | Characteristics |
|---|---|
| 0 | One MCT incompletely excised from dermis without lymph node involvement: a) without systemic signs b) with systemic signs |
| I | One MCT confined to the dermis, without lymph node involvement: a) without systemic signs b) with systemic signs |
| II | One MCT confined to the dermis with lymph node involvement: a) without systemic signs b) with systemic signs |
| III* | Large infiltrating MCT with or without lymph node involvement: a) without systemic signs b) with systemic signs |
| IV | Any tumour with distant metastasis, including blood or bone marrow involvement |
Location
In general, our ability to recognize MCT earlier and combine better surgical approaches with radiotherapy has resulted in overall better survival times for patients with MCT. However, certain locations may still warrant a more guarded long-term prognosis; in particular, aural (early metastasis to regional lymph nodes), subungual, perianal, oral and other mucocutaneous sites are associated with more undifferentiated tumours and a poorer prognosis, with metastasis earlier in the course of their disease (Cahalane et al 2004, Sfiligoi et al 2005, Thamm et al 2006). Any MCT at a mucocutaneous site is considered grade III disease (due to metastatic potential) no matter what grading they are given by the pathologist. Visceral disease carries a grave prognosis (O’Keefe 1990, Ozaki et al 2002).
Growth rate
This is determined by tumour volume/number of weeks the tumour was present. Low-grade MCT tend to have a long, stable history. Some MCT will remain sessile for weeks, months or even years before entering a phase of rapid growth; the biological trigger is unknown.
Any growing MCT should be removed immediately, as should any ‘quiescent’ MCT located in an area where growth would compromise surgical margins, e.g. distal extremity or face. Rapid growth is a negative prognostic indicator and the faster an MCT is growing, the sooner it should be addressed. Best practice is to diagnose and treat as early as possible. A ‘wait and see’ approach is potentially detrimental to the patient.
Systemic signs
Vomiting, melaena, anorexia, or widespread erythema and oedema due to massive mast cell degranulation are more often associated with large cutaneous MCT or disseminated disease (Mullins et al 2006, O’Keefe 1990, Pollack et al 1991). In 16 cases of visceral MCT, the median survival time (MST) was 90 days and all dogs died of MCT (O’Keefe 1990).
Recurrence
Recurrence following surgical excision is thought to carry a more guarded prognosis (Thamm et al 2006).
Breed
Boxers usually have well-differentiated tumours and therefore a better prognosis (Bostock 1986). Other breeds that have a predisposition to develop more biologically aggressive MCT include Bernese Mountain Dogs (BMD), Shar Pei and Bull Mastiff. Multiple cutaneous MCT are seen most frequently in Boxers, Golden Retrievers, Labrador Retrievers and Weimaraners.
Additional prognostic factors
In an attempt to better understand prognosis for patients with MCT, new indicators are constantly been evaluated.
The first of these was evaluation of argyrophilic nuclear organizer regions (AgNORs). AgNORs are an indirect measure of cell proliferation and require silver colloid staining of paraffin-embedded sections. The number of AgNORs has been correlated with histological grade and postsurgical outcome. The higher the count, the poorer the prognosis tends to be (Bostock et al 1989, Simoes et al 1994). The main problem with AgNORs is subjectivity; it is very operator dependent and not widely available.
Proliferating cell nuclear antigen (PCNA) is a 36 kD acidic non-histone protein required for DNA synthesis and is an immunochemical test that again is an indirect assessment of tumour cell proliferation (Abadie et al 1999, Simoes et al 1994).
Other cellular proliferation indices include Ki-67 and this, in combination with AgNORs and PCNA-positive nuclei, may indicate the likelihood of MCT recurrence after incomplete excision (Séguin et al 2006). BrdU LI (bromodeoxyuridine) is another marker of cellular proliferation correlated with tumour grade (Sakai et al 2002).
Intratumoral microvessel density correlates to invasiveness, mitotic rate and prognosis (Preziosi et al 2004). Microvessel density/angiogenesis was significantly higher in high-grade MCT compared to intermediate and low-grade MCT (Ranieri et al 2003).
Other markers currently being evaluated include c-kit mutation and high-grade tumours (Zemke et al 2002), Mdm2 expression (an immunohistochemical marker that plays a crucial role in mast cell tumorigenesis and is consistent with histological grade) (Wu et al 2006), and aberrant CD117 immunoexpression patterns and higher histological grade (Gil da Costa et al 2007).
Treatment
This depends on presence or absence of negative prognostic factors, and on the clinical stage of disease. Surgical excision with clean margins and marginal excision with adjuvant radiation therapy are the most successful treatment options to date.
Surgery
For tumours localized to the skin or subcutaneous tissues in areas amenable to wide excision, surgery is the treatment of choice.
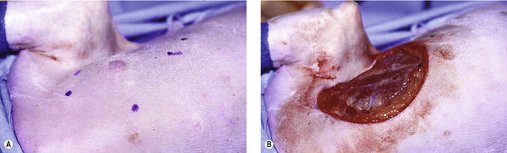
The surgeon should include a 3 cm margin of surrounding normal tissue. However, margins of 2 cm may be sufficient for low- and intermediate-grade MCT (Simpson et al 2004) (Figure 19.5). Wide margins are deep as well as lateral, and for a deep margin a fascial plane is required to ensure adequate excision (see Chapter 5). Histological assessment of margins is important. If planned curative surgery has not been achieved, then further local therapy may be warranted. This may entail a second excision with additional wide margins or adjuvant radiation therapy depending on MCT location. Not all incompletely resected MCT will recur, especially if low–intermediate grade and the possibility of normal mast cells at tissue margins may cause some confusion (Séguin et al 2006). However, if the resection is incomplete and recurrence would be difficult to deal with (distal extremity or face), adjuvant therapy is indicated.
Wide margins to ensure adequate excision of MCT mean that in many cases flaps are required (Figure 19.6). It is important that before a flap is attempted the surgeon is entirely confident that this will result in complete excision. If not, it is better for the patient to do a marginal excision and refer for radiotherapy. There is nothing worse than putting a patient through the long process of recovering from extensive surgery and then not having a margin, because at that point the radiation oncologist has to assume potential recurrence in any region of the flap. It also makes the whole process more expensive for the client than it needed to be.
For many patients the long-term prognosis with surgery alone is good. Patnaik et al (1984), in a large retrospective study, showed that 1550 days after surgery 93% of dogs with well-differentiated MCT were alive, compared with 44% with intermediate-grade tumours and only 6% with poorly differentiated tumours. A number of studies since then have come to similar conclusions concerning prognosis (Murphy et al 2004, Séguin et al 2006, Sfiligoi et al 2005).
MCT often arise in areas where it is not possible to achieve adequate surgical margins, e.g. the head and distal extremities. In the case of a MCT on a distal extremity, amputation of the leg is an option and in some cases can be the treatment of choice, especially if the tumour has extended 360 degrees around either the carpus or the tarsus. Usually, however, amputation is seen as a salvage procedure and in most cases the goal is to preserve the limb.
Regardless of local therapy, dogs with low–intermediate grade MCT should be re-evaluated regularly for local recurrence and regional spread.
For tumours where adequate margins are not achievable, e.g. tumours of the extremities, ensure that the grade is known via incisional biopsy before treatment.
Low–intermediate grade tumours
• Treatment of choice is marginal resection and radiation. Clinical stage 0 disease and follow-up with radiation produced 2-year control rates of 85–95% for stage 0 tumours of low or intermediate grade (Al-Sarraf et al 1996, Frimberger et al 1997, LaDue et al 1998, Turrel et al 1988). Prophylactic radiation of cytologically negative lymph nodes can be performed but definitive evidence for survival advantage is lacking (Poirier et al 2006).
• Limb amputation: wide margins but least functional outcome.
• External beam radiation alone: 40–50 Gy gives 1-year control of 50% (Turrel et al 1988). Coarse-fraction radiation (3–4 weekly, 8–10 Gy) gives local responses lasting 1 year or longer.
• Marginal resection and chemotherapy (alternate choice if radiation is not available) (Thamm et al 2006).
For patients with positive lymph nodes (determined on histopathology) adjuvant chemotherapy is advisable and the authors recommend three cycles of CCNU (lomustine) at intervals of 3 weeks with follow-up rechecks for lymph node and liver/spleen assessment at 3-monthly intervals for 1 year. In many patients with early metastasis to the lymph node, surgical excision plus chemotherapy may be sufficient to prevent further spread, so although these patients have a more guarded prognosis, regional involvement does not preclude definitive treatment for the primary tumour.
Poorly differentiated tumours
For any patient with distant metastasis or poorly differentiated tumours the overall prognosis is poor and treatment options may be palliative rather than definitive.
• The treatment of choice for poorly differentiated tumours without obvious metastases is surgery/radiotherapy for local control and chemotherapy for metastatic disease.
• For patients with obvious metastatic spread the value of extensive surgery is questionable except in cases when a lack of treatment for local disease would reduce quality of life for the patient. This can be a difficult decision for both the veterinary professional and the client, requiring that the client be informed of all the options.
• Palliative treatment would include chemotherapy or prednisolone (see below).
Radiotherapy
In veterinary medicine the most frequent application of radiotherapy is in the control of incompletely resected MCT. Radiotherapy should be considered in all patients where an MCT has been incompletely resected and where no further surgery can be carried out to obtain surgical margins. This means that the majority of patients eligible for radiotherapy have had MCT on the head/muzzle region or a distal extremity (Figure 19.7).
The decision as to the value of radiotherapy in an individual patient depends on the experience of the radiation oncologist. The rule of thumb used by the authors is that any fast-growing/aggressive MCT in an area of the body that would be difficult to re-excise should it regrow should receive radiation. Any MCT that has shown invasion onto tendons etc. should also be irradiated, irrespective of grade. MCT that may be best monitored are those of lower grade, less invasive or in areas that should there be recurrence, good options for further treatment are available.
Age and breed can also influence treatment decisions. The younger the patient, the better it is to avoid radiotherapy for MCT, if possible, because of the potential for long-term side effects of treatment (see Chapter 7).
A number of treatment protocols are available in the literature but all give good long-term control for stage 0 tumours of low to intermediate grade (median 2-year control rates of 85–90%). Radiotherapy is equally successful in local control of high-grade MCT but unfortunately these patients fail early due to metastatic disease.
Radiotherapy can also be used to shrink tumours before surgery and actually sterilizes the margins so that these are not underestimated at the time of surgical excision.
Radiotherapy can be used as the sole treatment in the management of MCT. In this scenario, it should be considered palliative therapy and is most often applied to MCT located on the muzzle not amenable to surgery or the client declines surgery due to the potential cosmetic appearance, e.g. MCT of the distal extremities that are not amenable to surgery and the client declines amputation. The major concern in irradiating bulky MCT is mast cell degranulation and the resulting consequences of acute hyperhistaminaemia.
Large MCT can be considered for radiotherapy to shrink them down to improve quality of life. However, the potential for complications, as above, is high, and such patients require hospitalization after treatment.
Chemotherapy
The role of chemotherapy remains an area of discussion in veterinary medicine. The primary treatment modalities are undoubtedly surgery and radiotherapy. However, in patients with metastatic disease (Figure 19.8) or multiple cutaneous MCT, surgery and radiotherapy may not be appropriate. In general, for multiple cutaneous MCT, multiple marginal excisions are combined with adjuvant chemotherapy.