Canine mammary tumours
Mammary tumours are the second most common tumours of dogs (second to skin tumours) (Moulton 1999) (Figure 17.1). Mammary tumours are still frequently encountered in countries were ovariohysterectomy (OHE) or ovariectomy is not routinely performed on young female dogs that are not acquired for breeding purposes. A number of studies have shown that early OHE protects female dogs from developing mammary cancer in later life. The risk of developing mammary cancer if spayed prior to first heat is 0.05%, 8% after 1st, and 26% after 2nd, compared to intact dogs (Schneider et al 1969). OHE after four or more cycles or greater than 2.5 years of age has little or no protective effect on the development of malignant mammary tumours (Misdorp 1988, Schneider et al 1969). It is therefore a preventable condition and as such client counselling and veterinary surgeon awareness will continue to reduce the incidence of this preventable cancer.
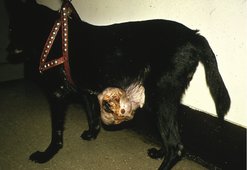 |
| Figure 17.1 (Courtesy S Withrow.) |
Pathogenesis
The correlation with protection against developing mammary tumours and OHE indicates priming of the mammary tissue under hormonal influence. Prolonged, dose-related exposure to synthetic progestins has been shown to induce proliferation of mammary epithelial cells, potentially leading to genetic errors that may subsequently result in the development of mammary tumours (benign and malignant) (Misdorp 1991, Støvring & Glattre 1997). Administration of diethylstilboestrol is associated with ovarian tumour development but not mammary (Jabara, 1962a and Jabara, 1962b).
The exact mechanisms and interactions at the cellular level that lead to the development of malignant mammary tumours are unknown. Hormonal therapy (anti-oestrogen drugs such as tamoxifen) has been shown to delay the onset of metastatic disease in humans with oestrogen receptor-positive tumours. The presence of oestrogen, progesterone and prolactin receptors on canine mammary tissue and tumours has been evaluated (Rutteman & Misdorp 1993). Only 50% of malignant primary tumours were positive for these receptors and they were infrequently detected on metastatic lesions.
The role of dietary factors in the development of canine mammary tumours is undetermined although one study did indicate that obesity at 1 year of age may lead to an increased risk of developing mammary neoplasia (without consideration of OHE) (Pérez-Alenza et al 1998).
Clinical signs
The presence of a mass associated with the mammary tissue warrants investigation. The caudal mammary glands are said to be more frequently involved than the cranial glands, and tumours can present as either isolated lumps or multiples. In the case of multiple lumps, each one must be treated as an individual. Usually, these lumps are non-painful; they may appear and remain static or grow rapidly. Cystic lesions can also be present in the mammary tissue, but care is required when evaluating such lesions as there may be underlying tumour associated with what appears cytologically to be cystic fluid.
Evaluation of the patient
Signalment
Most mammary tumours arise in middle-aged intact females with a peak incidence between 6 and 10 years of age (Egenvall et al 2005). Mammary cancer in males is rare (<1% of all mammary tumours occur in males) (Lana et al 2007), and female dogs are 62 times more likely to develop mammary gland tumours than male dogs (Saba et al 2007). In a recent study of eight male dogs with mammary tumours, seven out of eight tumours were benign, for which surgery alone provided long-term control (Saba et al 2007). Affected male dogs may have an oestrogen-secreting Sertoli cell tumour of the testis (Moulton 1999).
Mammary neoplasia is rarely seen in young dogs (Egenvall et al 2005); however, benign cystic hyperplasia can be seen in dogs involving all mammary glands. This is typically seen in dogs during metoestrus, pregnancy or after treatment with progestins. This usually resolves without surgical intervention (Rutteman & Kirpensteijn 2003).
History
It is important to ascertain the reproductive status of the patient, the stage of oestrus cycle, the time the lump has been present and if it has grown since first noticed.
Physical examination
A good physical examination is required. The mammary lump should be palpated for size, whether fixed to underlying tissue or freely movable, any enlargement of the draining lymph nodes, and any ulceration or oedema. These findings, coupled with the client’s perception of rate of growth, are all indicators of potential malignancy.
Cytology
Fine needle aspiration (FNA) cytology to differentiate benign mammary adenoma from malignant carcinoma is generally unhelpful, due to mixed cell populations within mammary tumours.
Biopsy
Incisional biopsies give more information than cytology and may be useful to assist in staging and surgical planning; however, excisional biopsies are often more appropriate to provide diagnosis, prognosis and treatment in one step (see surgical treatment below). If an incisional biopsy is taken it is still important to submit the final specimen for evaluation by a pathologist; more than one benign biopsy has been shown to have a malignant component on final histopathology.
Staging the patient
Approximately 50% of mammary tumours are benign adenomas that are curable with adequate surgery (Brodey et al 1983, Gilbertson et al 1983). A large percentage of mammary carcinomas are low grade and carry a fair prognosis with adequate excision. The prognosis for non-invasive carcinoma is very good (Kurzman & Gilbertson 1986). Aggressive carcinomas are seen less frequently but warrant a guarded prognosis (Kurzman & Gilbertson 1986). Inflammatory carcinomas warrant a very poor prognosis, with a median survival time (MST) of 25 days with palliative care (Peña et al 2003, Pérez-Alenza et al 2001).
For a patient with suspected malignant mammary cancer, staging is important. The tumour/node/metastasis (TNM) system proposed by the World Health Organization (WHO) is typically used to stage veterinary patients with mammary tumours (Table 17.1).
| Tumour | Regional lymph node (LN) | Distant metastasis |
|---|---|---|
| T0: no measurable tumour | N0: no LN metastasis | M0: no distant metastasis |
| T1: <3 cm | N1: LN metastasis | M1: distant metastasis |
| T2: 3–5 cm | ||
| T3: >5 cm | ||
| T4: inflammatory Ca | ||
| a: not fixed | a: not fixed | |
| b: fixed | b: fixed |
1. Establish a minimum database of routine biochemistry, haematology and urinalysis.
2. Physically check the draining lymph nodes for any enlargement. If palpable, FNA of the regional lymph node should be taken and evaluated for neoplastic cells.
3. Thoracic radiographs, good quality right and left laterals are required to assess for metastatic disease. CT may be of more use for the detection of pulmonary metastatic disease from mammary gland neoplasia, as radiographs are not very sensitive, detecting metastasis in only one of six dogs with necropsy-confirmed intrathoracic metastasis (Baumann et al 2004). Furthermore, CT has been shown to be more sensitive than radiographs for detecting pulmonary metastases (Nemanic et al 2006). Clinical and histological staging of mammary tumours are shown in Table 17.2a and Table 17.2b.
| After Owen (1980). | |||
| Clinical stage | Tumour | Regional lymph node | Metastasis |
|---|---|---|---|
| I | T1 | N0 | M0 |
| II | T0, T1 | N1 | M0 |
| T2 | N0, N1 | M0 | |
| III | T3 | N0, N1 | M0 |
| T0–T3 | N1 | M0 | |
| IV | T0–T3 | N0, N1 | M1 |
| V | T4 | N0, N1 | M1 |
| After Gilbertson et al (1983). | |
| Stage | |
|---|---|
| 0 | Tumour cells limited to ductal tissue |
| I | Tumour cells invading stromal tissue |
| II | Vascular/lymphatic invasion, regional lymph node metastasis |
| III | Distal metastasis present |
Poor prognostic signs
• Inflammatory (Peña et al 2003, Pérez-Alenza et al 2001)
• Size (<3 cm better than >3 cm) (Bostock 1986, Chang et al 2005, Kurzman & Gilbertson 1986, Philibert et al 2003)
• Node positive (for stage, see Table 17.2b) (Chang et al 2005, Gilbertson et al 1983, Hellmén et al 1993, Karayannopoulou et al 2005, Kurzman & Gilbertson 1986, Yamagami et al 1996)
• Histological type and grade (Benjamin et al 1999, Chang et al 2005, Gilbertson et al 1983, Hellmén et al 1993, Karayannopoulou et al 2005, Kurzman & Gilbertson 1986, Misdorp et al 1971, Peña et al 2003, Pérez-Alenza et al 2001)
• Invasiveness (including fixation to underlying tissue) (Gilbertson et al 1983, Hellmén et al 1993, Kurzman & Gilbertson 1986)
• Presence of distant metastasis (Chang et al 2005)
• Degree of nuclear differentiation (Gilbertson et al 1983, Karayannopoulou et al 2005)
• Evidence of lymphoid cellular reactivity in tumour vicinity (Gilbertson et al 1983)
• Intravascular growth, steroid hormone receptor activity (Geraldes et al 2000, Nieto et al 2000)
• S-phase fraction (Hellmén et al 1993)
• High Ki-67 proliferation indices (Zuccari et al 2004)
• DNA aneuploidy (Hellmén et al 1993)
• Number of argyrophilic nuclear organizer regions (AgNORs) (Bostock et al 1992)
• Higher angiogenesis (Restucci et al 2000)
• Tumours present for >6 months had a higher risk of lymph node metastasis (Chang et al 2005).
Non-prognostic signs
• Gland involved (Schneider et al 1969)
• Type of surgery (MacEwen et al 1985)
• Spaying at time of treatment (some controversy but needs more study) (Chang et al 2005, Morris et al 1998, Sorenmo et al 2000, Yamagami et al 1996)
• Multiple lumps (Benjamin et al 1999, Moulton et al 1986).
Malignant mammary tumours
Whilst approximately 50% of canine mammary tumours are benign, between 20 and 40% are considered to be malignant and can arise from different structures within the mammary tissue (Brodey et al 1983, Gilbertson et al 1983).
Carcinomas
Mammary carcinoma (adenocarcinoma) is the most common malignant tumour and is variously described as solid, tubular or papillary. They can be simple (epithelium alone) or complex (epithelium and myoepithelium). The infiltrative characteristics of the tumour in conjunction with the degree of invasion into local lymphatics and capillaries are important in determining the long-term prognosis regarding local recurrence and metastatic potential. The most aggressive carcinomas have lost their identifying characteristics and are described as anaplastic or poorly differentiated tumours; the latter warrant a guarded prognosis.
Histological grade 0 has a 19% recurrence compared to 97% for grade II (Gilbertson et al 1983). Also similar with nuclear differentiation, poorly differentiated had 90% recurrence, 68% with moderate and 24% with well differentiated (Gilbertson et al 1983). The most common metastatic sites for mammary carcinomas are the draining lymph nodes, followed by the lungs. Bone, liver or brain metastases are less frequent in veterinary patients compared to human patients (Lana et al 2007).
Inflammatory carcinomas
• Severe and rapid spread
• Red, hot, painful, pruritic
• Spreads laterally down legs and across midline
• Inoperable
Inflammatory carcinomas are rare neoplasms with an extremely poor prognosis. These tumours can often be identified at the time of presentation. Typically, physical examination reveals a firmly attached mass that is painful, erythematous and warm. It may extend to involve multiple glands and cause oedema of the peripheral limb (Figure 17.2). These patients are typically in chronic disseminated intravascular coagulation (DIC).
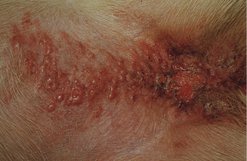 |
| Figure 17.2 (Reproduced from Small Animal Clinical Oncology 3rd edition, S Withrow & E G MacEwan, 2001, with permission from Elsevier.) |
Typically thoracic radiographs are unremarkable on presentation, but in the few patients that survive greater than 60 days pulmonary metastases will become apparent. In those cases where surgical intervention has been attempted, the complication rate was high due to the rapid recurrence and regional spread of tumour. Histology on these tumours shows significant infiltration of neoplastic cells into the lymphatics, explaining the clinical presentation of oedema.
Anecdotally, radiotherapy as a palliative procedure may improve quality of life for a short period of time in certain patients but the overall prognosis for any patient with inflammatory mammary carcinoma is poor (Peña et al 2003, Pérez-Alenza et al 2001).
Malignant mixed tumours
These arise from both the epithelial and connective tissue components of the mammary gland. They are also known as ‘carcinosarcomas’ and are relatively uncommon.
Other mammary tumours
• Sarcomas: These are rare tumours of the mammary gland and the origin within the mammary tissue is unknown. Sarcomas are considered to have poor prognosis. Most dogs die within 9–12 months (Hellmén et al 1993, Misdorp et al 1971).
• Mast cell tumours: These should be managed as with any mast cell tumour (see Chapter 19).
• Lymphoma: Lymphoma has been reported as an isolated mass in the mammary tissue as a rare occurrence.
Treatment
Surgery
Important features are size and invasiveness or adherence. The treatment of choice is surgical excision with appropriate margins. No clinical trial has shown improvement in survival with radical versus local removal.
OHE when mammary tumours are removed does not have a significant effect on the progression of malignant disease (Morris et al 1998, Yamagami et al 1996). In another study, however, OHE at the time of mammary tumour removal improved survival 2 years after surgery, and was more beneficial for complex carcinomas than for simple carcinomas (Chang et al 2005). Sorenmo et al (2000) also found a beneficial effect of OHE at the time of mammary tumour removal. One-quarter of dogs with benign mammary tumours developed another mammary tumour within 2 years, whether they were spayed or not (Morris et al 1998). OHE helps prevent the development of further benign mammary tumours.
Goal of surgery
Remove the entire tumour by the simplest procedure (Figure 17.3). More surgery is not better surgery.
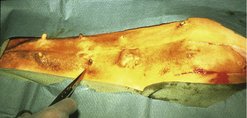 |
| Figure 17.3 (Courtesy S Withrow.) |
Surgical procedures
• Lumpectomy: Lumpectomy is performed for small nodules <0.5 cm that are firm and superficial. Incomplete margins are acceptable for benign lesions but if malignant, re-excision to achieve clean margins is warranted.
• Mammectomy: Mammectomy to remove the whole gland is used for centrally located tumours, >1 cm, or with any degree of fixation to skin. Skin and abdominal wall fascia should be removed if involved. For malignant lesions, margins of 1–2 cm of grossly normal tissue are generally adequate.
• Regional mastectomy: As above, with several glands removed together for ease of surgery (e.g. glands 1, 2 and 3 together or glands 4 and 5 together). The inguinal lymph node is usually removed en bloc with glands 4 and 5. The axillary lymph node is only removed if enlarged or cytologically positive for metastasis.
• Unilateral (1–5) mastectomy: Performed to achieve multiple lumpectomies with greater ease and rapidity. It does not improve survival compared with multiple lumpectomies or mammectomies (MacEwen et al 1985). In dogs, there is minimal need for a bilateral (radical) mastectomy. There is usually also minimal loose skin to allow this to be achieved with clean margins. A better approach would be to do staged unilateral mastectomies, ensuring adequate margins are achieved with each surgery. As 50% of canine mammary tumours are benign, it is important to not perform a ‘malignant’ surgery for a benign disease. More surgery, if required, can be done later.
• Bilateral (radical) mastectomy: This entails considerable morbidity, time and money and does not change survival, compared to multiple mammectomies/lumpectomies.
Other treatments
Chemotherapy
For patients with aggressive mammary tumours exhibiting lymphatic or vascular invasion there is a high rate of local recurrence and metastasis. However, the role of adjuvant chemotherapy in these patients has not been adequately determined. Single-agent doxorubicin (Hahn et al 1992), doxorubicin and cyclophosphamide, and doxorubicin and docetaxel (Simon et al 2006) are chemotherapy protocols that have been used for veterinary patients with aggressive mammary carcinomas. Anecdotally, some patients are reported to have benefited from doxorubicin-based chemotherapy but prospective studies are few in number and have shown equivocal benefit from adjuvant chemotherapy. Local recurrence can be re-addressed by surgery and a second surgical procedure should be considered if the margins are not adequate. Chemotherapy should be reserved for patients at risk from metastases.
Radiotherapy
Radiotherapy is rarely used in the management of canine mammary cancer, but undoubtedly has a role for patients with locally recurrent disease that is not amenable to further surgery or for patients with regional metastasis to lymph nodes also not amenable to surgery. In such cases, radiation would be considered a palliative treatment.
Hormonal therapy
For patients diagnosed with mammary carcinoma, no benefit has been shown to carrying out OHE at the time of mastectomy (Morris et al 1998). This was to be expected due to the low levels of receptors identified on mammary tumours. In human patients anti-oestrogen drugs such as tamoxifen are effective in delaying the development of metastatic disease in those patients with oestrogen-positive tumours. No such benefit has been noted in canine patients and the oestrogenic effect of tamoxifen on the uterus resulting in pyometra or stump pyometra is an undesirable side effect. However, this drug may benefit the small number of dogs that have tumours that are oestrogen receptor positive but to establish the benefit a prospective trial would be required on suitable candidates (Morris et al 1993). At the present time there is little indication that hormonal therapy is of benefit to canine patients.
Tumours of the uterus
Neoplasia of the uterus is uncommon in dogs, primarily because of the large percentage of dogs that undergo elective OHE. Most uterine tumours encountered in dogs are of mesenchymal origin (85–90% benign leiomyomas, 10% leiomyosarcomas) (Theilen & Madewell 1979). Other malignant tumours of the canine uterus sporadically reported include carcinoma (Cave et al 2002, Murakami et al 2001, Payne-Johnson et al 1986, Pena et al 2006, Vos 1988).
Clinical signs
Clinical signs are non-specific and these tumours may be an incidental finding. In some cases a mass can be palpated, or a vaginal discharge may be present.
Imaging
Abdominal radiographs may show the presence of a soft tissue mass consistent with the uterus. Abdominal ultrasound is useful as this allows better delineation of tumour and assessment of the regional lymph nodes for evidence of metastasis.
Treatment and prognosis
The treatment of choice is OHE (Figure 17.4). The value of chemotherapy or radiotherapy is unknown.
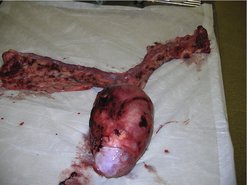 |
| Figure 17.4 |
In general, the prognosis for dogs with uterine tumours is good as the majority are benign.
Tumours of the vagina and vulva
Tumours of the vagina and vulva are seen with greater frequency than tumours of the ovary or uterus. Most of these tumours are of mesenchymal origin (Table 17.3).
| Benign | Malignant |
|---|---|
• Leiomyoma • Fibroma • Fibroleiomyoma • Lipoma | • Leiomyosarcoma • Adenocarcinoma • Fibrosarcoma • Transmissible venereal tumour (TVT) |
Leiomyomas are typically seen in older (10–11-year-old) intact females and account for approximately 85% of all vaginal and vulval tumours in dogs (Kydd & Burnie 1986, Thacher & Bradley 1983). Production of oestrogen is associated with growth of these tumours. The malignant variant, leiomyosarcoma, is the most common malignant tumour of the vagina and vulva. Leiomyosarcomas are locally invasive but are slow to metastasize.
Other tumours reported in this region are rhabdomyosarcomas, mast cell tumours, haemangiosarcoma, squamous cell carcinoma (SCC), adenocarcinoma, epidermoid carcinoma, osteosarcoma and transmissible venereal tumours (Brodey & Roszel 1967, Herron 1983, Hill et al 2000, Suzuki et al 2006, Thacher & Bradley 1983, Theilen & Madewell 1979).
Clinical signs
These are usually associated with the size and position of the mass as these tumours can be either extra- or intraluminal growths. Patients can present with a bulging of the perineum, prolapse of tumour from the vulva, dysuria, stranguria, haematuria, vulval bleeding or discharge, or tenesmus.
Physical examination
An obvious swelling or protruding mass from the vulva may be visible on examination of the perineum and confirmation of location of the mass can be obtained via digital examination per rectum or per vagina.
Diagnostic work-up
Routine blood work is usually unremarkable; however, anaemia, hypoproteinaemia and thrombocytopenia may occur due to chronic blood loss, and hypoglycaemia is a possible paraneoplastic syndrome. Secondary infections may also occur, causing a leucocytosis.
Imaging
Radiographs and ultrasound have limited application due to the intrapelvic location of these tumours. Contrast radiographic studies including positive contrast retrograde vaginourethrography are useful to determine the size and extent of the primary. Chest radiographs are indicated to rule out pulmonary metastases. Advanced imaging such as a CT scan may be beneficial.
Treatment
Surgical resection is complicated by the vascularity of the vagina and vulva, and the neovascularization that occurs with tumour growth. Blood loss during surgery can be considerable. The surgeon must obtain a packed cell volume (PCV) and total protein prior to surgery, monitor for intraoperative blood loss and have an ability to provide a blood transfusion if needed.
Vaginal leiomyomas and leiomyosarcomas are approached surgically via the perineum. If there is any evidence of a haemorrhagic or purulent vaginal discharge, or the tumour is palpable via the vagina (intraluminal), the tumour is expected to involve the inner layers of the vaginal wall and an episiotomy may be needed to facilitate exposure.
If the tumour is pedunculated and intraluminal, it may be removed after ligation with a transfixing suture. If it is more broad based it may be amenable to a debulking surgery or ‘shelling- out’, which is only appropriate for benign or histologically low-grade tumours.
If there is no vaginal discharge, and the tumour is palpated (by a combination of rectal and vaginal examination) to be arising from the outer vaginal wall (extraluminal), an episiotomy may allow adequate visualization; alternatively, a perineal approach to the dorsal, ventral or lateral vagina may be preferable.
If a preoperative incisional biopsy has shown a malignancy, e.g. an aggressive sarcoma, a squamous cell carcinoma or adenocarcinoma, a full thickness resection of the vaginal wall and tumour en bloc, followed by anastomosis of grossly normal vaginal wall (if possible) is required. The surgeon should ensure adequate exposure to confirm there is no iatrogenic damage to the urethra, and the urethra should be catheterized prior to surgery to aid intraoperative identification.
An incisional biopsy should always be performed prior to extensive surgical resections to allow the surgeon to best plan surgery to obtain a potential cure. A wider resection, such as vulvovaginectomy and perineal urethrostomy (Bilbrey et al 1989), may provide a cure or improved palliation for a malignant vaginal/vulval tumour, and attempts at surgical cure ought not to be thwarted by an extensive but ‘dirty’ surgery.
As leiomyomas, fibromas, polyps, leiomyosarcomas and most other vaginal tumours are hormone dependent, surgical excision of the vaginal tumour should be accompanied by OHE to prevent recurrence. The prognosis for adenocarcinoma and squamous cell carcinoma is poor due to metastasis or local recurrence, whereas benign or low-grade mesenchymal lesions have a good prognosis with treatment.
Tumours of the ovary
Ovarian tumours are rare in the dog, primarily because most dogs undergo OHE at a relatively young age and, when present, are typically seen in middle to older aged dogs.
Cystadenomas/carcinomas are the most common tumours encountered and account for approximately 50% of ovarian tumours (Nielsen et al 1976, Patnaik & Greenlee 1987). They can be either unilateral or bilateral. They arise from the surface epithelial layer of the ovary and malignant tumours metastasize to para-aortic lymph nodes, kidneys, omentum, liver and lungs. They will also seed tumour throughout the abdominal cavity, resulting in carcinomatosis and formation of a malignant effusion.
Granulosa cell tumour develops from the gonadostromal (sex cord) tissue of the ovary. These tumours are usually unilateral and about 20% are malignant with a similar metastatic pattern as seen with cystadenocarcinomas (Hayes & Harvey 1979, Herron 1983, Neilsen et al 1976, Patnaik & Greenlee 1987, Theilen & Madewell 1979). Granulosa cell tumours may be hormonally active and secrete oestrogen, and comprise 50% of ovarian tumours in some reports (Dow 1960, Herron 1983, Neilsen et al 1976, Patnaik & Greenlee 1987).
Other sex cord stromal tumours include the extremely rare and benign tumours thecoma and luteoma.
The third category of ovarian tumours includes the germ cell tumours – dysgerminoma and teratoma/teratocarcinoma. Dysgerminomas arise from undifferentiated germ cells and are considered to be malignant in that metastasis occurs to regional lymph nodes and other abdominal organs in approximately 10–30% of cases (Andrews et al 1974, Dehner et al 1970, Neilsen et al 1976). Teratomas are usually, but not always, benign and are often composed of two or three germinal layers including bone, cartilage, brain or glandular epithelium.
Clinical signs
Many tumours are incidental findings at the time of an elective OHE. However, an ovarian tumour should be suspected in any intact female with abnormal oestrus cycles. Signs consistent with excess oestrogen production may indicate the presence of a granulosa cell tumour.
Presenting signs may include prolonged oestrus, alopecia, mammary hyperplasia, cystic endometrial hyperplasia/pyometra, vaginal discharge, vulval swelling, attractiveness to males and very occasionally myelosuppression due to hyperoestrogenism. Other clinical signs that may be apparent include lethargy, abdominal distension, weight loss, presence of a palpable abdominal mass and lumbar pain.
Physical examination
Abdominal palpation may indicate the presence of an abdominal mass.
Diagnostic work-up
In patients with chronic hyperoestrogenism there may be evidence of bone marrow suppression on haematology. This is sometimes irreversible, even with removal of the primary tumour.
Imaging
Plain radiographs, including thoracic films, and ultrasonography will help define the presence and location of the mass and, especially with ultrasound, the possible presence of metastases.
Cytology
For patients with peritoneal effusion cytology is valuable to determine the presence of malignant cells.
Treatment
The treatment of choice for all patients that do not show extensive signs of metastasis is OHE. For those patients with benign tumours the prognosis is good. In patients with malignant tumours with evidence of regional spread the efficacy of chemotherapy has not been proven. Anecdotally, canine patients with carcinomatosis have responded to intracavitary cisplatin chemotherapy; however, as in humans, the effectiveness of treatment depends on overall tumour burden and patients with large intra-abdominal masses (>0.5 cm in diameter) do not respond as well as patients with small nodules or neoplastic cells present in the effusion.
Ovarian carcinoma is rare in dogs, but as in humans the potential for local dissemination throughout the abdominal cavity is high.
Feline mammary tumours
Mammary tumours are the third most common neoplasm described in the cat after skin tumours and lymphoma/leukaemia (Carpenter et al 1987, Dorn et al, 1968a and Dorn et al, 1968b, Hayes et al 1981). As with dogs, mammary tumours primarily affect female cats with an extremely low incidence in males. Also as with dogs, hormonal influences are believed to contribute to the development of mammary tumours as intact cats and cats treated with progestins have an increased risk of developing mammary tumours (approximately 10% risk of cancer with progesterone therapy). All glands are at equal risk (Anderson & Jarrett 1966, Hayden & Neilson 1971, Hayes 1977, Weijer & Hart 1983). Feline mammary tumours have been shown to express fewer steroid hormone receptors when compared to normal feline mammary tissue (Hamilton et al 1976, Hayden et al 1981).
Studies have shown that cats undergoing OHE prior to 1 year of age have a significantly reduced incidence of mammary carcinoma (Overley et al 2005). Early spaying is protective but not as absolute as in the dog. Parity has not been shown to be a risk factor in the development of feline mammary tumours (Misdorp 1991, Overley et al 2005).
Signalment
Most mammary tumours occur in middle-aged to older female cats, with a median age of 10–12 years. Siamese cats may be at higher risk of developing mammary tumours than other breeds and these are often bilateral (Kessler & von Bomhard 1997).
Clinical signs
Clinical signs are of a mass associated with the mammary gland that may be attached to underlying tissue or ulcerated. In some cases the tumour may be plaque-like in appearance. No other clinical signs may be apparent.
Diagnostic work-up
As these are usually older patients, routine biochemistry, haematology and urinalysis should be carried out.
Physical examination should include palpation of the regional lymph nodes. An aspirate of the nodule helps to rule out any other cause of a mammary-associated lump.
Chest radiographs are indicated (left and right lateral views) to rule out metastatic lesions. In cats with metastases the typical thoracic radiograph is that of an interstitial military pattern; pleural effusion can also be present (Figure 17.5). The presence of a malignant effusion warrants a poor prognosis.
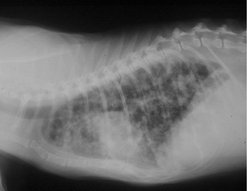 |
| Figure 17.5 (Courtesy S Withrow.) |
Malignant mammary tumours
In the cat >85% of mammary tumours are malignant; the majority of these are carcinomas (Bostock 1986, Carpenter et al 1987, Hayes et al 1981) (Figure 17.6).
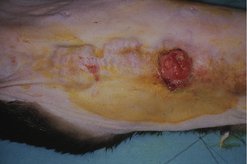 |
| Figure 17.6 (Courtesy S Withrow.) |
Staging
Because the majority of feline mammary tumours are malignant, staging is important and the WHO staging system for domestic animals recognizes four stages (Table 17.4). A number of studies have shown that the size of the primary at initial presentation is a good prognostic indicator and the staging system is based on tumour size (Hayes & Mooney 1985, MacEwen et al 1984, Weijer & Hart 1983).
| Stage | Tumour diameter |
|---|---|
| I | <1 cm |
| II | 1–3 cm |
| III | >3 cm |
| IV | Distant metastases |
The most significant factor affecting long-term survival in cats with mammary carcinoma is tumour size. For tumours <2 cm, MST is >3 years, compared to 15–24 months for 2–3 cm lesions and 4–12 months for lesions >3 cm (Ito et al 1996, MacEwen et al 1984, Viste et al 2002).
Stage at the time of presentation is also prognostic, cats with stage I and early stage II disease having a longer MST (>3 years) than cats with stage II–III (MST >2 years) or late stage III–IV (MST 6 months) (MacEwen et al 1984). Extent of surgery is also prognostic, with local recurrence in two-thirds of cats with conservative resection (MacEwen et al 1984). Bilateral mastectomy resulted in an MST of 917 days, compared to 428 days for regional mastectomy and 348 days for unilateral mastectomy (Novosad et al 2006).
Treatment
Surgery
Radical surgery is indicated in the cat with mammary neoplasia. Unlike the dog, radical mastectomy has a better prognosis than lumpectomy, and more aggressive surgery improves overall survival (MacEwen et al 1984, Novosad et al 2006).
Radical (bilateral) mastectomy and bilateral inguinal lymphadenectomy are generally performed up front at the first surgery, as cats have more loose skin than dogs, and cats are therefore more amenable to this surgery (Figure 17.7). However, if preferred by the surgeon or the client, staged unilateral mastectomies may be performed (separated by 2 weeks), as this may be less traumatic for the cat. Bilateral (radical) mastectomies create a large wound, and care should be taken to avoid hypothermia. Pain management should also be accordingly aggressive, as should postoperative supportive care.
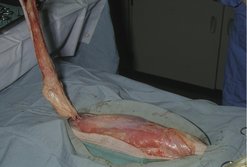 |
| Figure 17.7 (Courtesy S Withrow.) |
Mastectomy may be an appropriate palliative treatment, even in the cat with known metastasis, as ulcerated, bleeding, infected mammary cancers may cause significant morbidity, compared to pulmonary metastatic disease, which may be asymptomatic.
Chemotherapy
The role of adjuvant chemotherapy, either doxorubicin alone or doxorubicin in combination with cyclophosphamide, may have some benefit in prolonging survival in patients with late stage II to stage III disease, but large prospective studies have not been carried out to optimise chemotherapeutic protocols. The same combinations have been used in patients with inoperable disease where a 50% partial response rate was observed, and in those cats that did respond to chemotherapy the MST increased from 75 to 150 days.
The standard dose of doxorubicin was 1.0–1.1 mg/kg every 3 weeks for five treatments. The major side effect was mild anorexia at the lower dose; increasing the dose to 1.1 mg/kg is likely to result in more profound anorexia. Myelosuppression was generally not encountered (North & Mauldin 1997).
Radiotherapy
Radiotherapy is rarely used in the management of feline mammary tumours but may be of some benefit in those patients with inoperable tumours.
Feline mammary hypertrophic fibroadenoma complex (Figure 17.8)
This progesterone-dependent complex usually presents in young intact cats. It is induced in male and female neutered cats with exogenous progesterone.
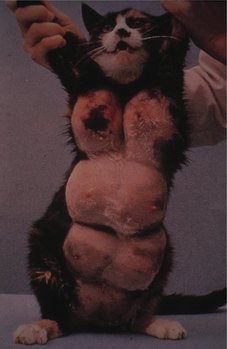 |
| Figure 17.8 (Reproduced from Small Animal Clinical Oncology 4th edition, S Withrow & D Vail, 2007, with permission from Elsevier.) |
Treatment is by OHE, usually via a flank approach to avoid the enlarged mammary glands.
Tumours of the ovary
Ovarian tumours are rare in the cat, accounting for only 3% of feline tumours. The reason for such a low incidence of ovarian cancer is probably related to the fact that most cats undergo OHE at a young age. The most common ovarian tumour seen in the cat is granulosa cell tumour, with clinical signs of paraneoplastic hyperoestrogenism common (Gelberg & McEntee 1985, Norris et al 1969).
Tumours of the uterus and cervix
Tumours of the uterus and cervix are uncommon in the cat. The most common malignant uterine tumour in the cat is adenocarcinoma (Miller et al 2003, O’Rourke & Geib 1970). Feline uterine sarcoma has also been reported (Cooper et al 2006, Miller et al 2003, Sato et al 2007).
Tumours of the vagina and vulva
These tumours are extremely rare in the cat; leiomyomas and fibromas have been reported.
TUMOURS OF THE MALE GENITAL TRACT
Tumours of the testicle
Tumours of the testicle are the most common tumours of the male genital tract, but as with mammary tumours in females are completely preventable by castration of male dogs not acquired for breeding purposes. One recent report found one or more testicular tumours in 27% of 232 necropsied dogs (Grieco et al 2008). Currently, these tumours account for 90% of all tumours of the male reproductive tract (Cotchin 1960, Hayes & Pendergrass 1976, von Bomhard et al 1978). They are typically seen in older male dogs, median age 10 years.
Cryptorchid dogs and dogs with inguinal hernias are at higher risk of developing testicular tumours and at an earlier age. Cryptorchid testes are 14 times more likely to develop neoplasia than scrotal testes, and dogs with inguinal hernias have a five times greater risk of testicular tumours (Hayes & Pendergrass 1976). About 50% of Sertoli cell tumours and about 30% of seminomas are in cryptorchids (Reif & Brodey 1969). The three most common testicular tumours are the Sertoli cell tumour, the interstitial cell tumour (Leydig) and the seminoma, each occurring with approximately equal frequency (Cotchin 1960). It is possible to get a combination of more than one type in one dog.
Clinical signs
The presence of an enlarged testicle or a mass within the testicle seen on routine examination is indicative of a testicular tumour. An abdominal mass may also be palpable, and testicular masses may be found on abdominal or testicular ultrasonography. Feminization syndrome may be the presenting clinical sign. An abdominal ultrasound is indicated to rule out a testicular tumour in any cryptorchid adult male dog.
Sertoli cell tumour (SCT)
Breed predisposition has been noted in Norwegian Elkhound, Fox Terrier, Afghan Hound, West Highland White Terrier, Airedale, Weimaraner, Pekingese and Shetland Sheepdog (Cooley & Waters 2001).
Clinical signs
About 50% of SCTs are found in cryptorchid testicles (Reif & Brodey 1969). SCTs can affect the ratio of oestrogen:testosterone (Mischke et al 2002). This ratio may be proportional to tumour size. The degree or presence of male feminization syndrome may depend on testicle location, with 16% of scrotal, 50% of inguinal and 70% of abdominal SCTs causing feminization syndrome (Lipowitz et al 1973). Oestrogen is bone marrow suppressive, and causes myelotoxicosis in 15% of SCTs with male feminization syndrome.
Clinical signs of male feminization syndrome include non-pruritic, symmetrical alopecia (beginning in the genital and perineal region and spreading to the ventral abdomen, thorax, flanks and neck), hyperpigmentation, gynaecomastia, galactorrhoea, penile atrophy and a pendulous prepuce, attractiveness to other males and standing in a female posture to urinate, symmetrical and squamous metaplasia of prostate, and atrophy of non-neoplastic testes.
Pathology
About 10–15% of SCTs are malignant (Fan & de Lorimier 2007), with metastasis to the inguinal, iliac and sub-lumbar lymph nodes as well as the lungs, liver, spleen, kidneys and pancreas. SCTs grow expansively to compress and destroy parenchyma.
Oestrogen myelotoxicosis often appears as an initial transient increase in granulopoiesis and a neutrophilic leucocytosis, followed by eventual hypoplasia of all cell lines and development of pancytopenia (thrombocytopenia, anaemia, granulocytopenia) (Sanpera et al 2002).
Diagnostic work-up and staging
This comprises abdominal and testicular palpation, rectal palpation, abdominal ultrasound, radiographs (abdominal and thoracic), exploratory abdominal surgery, haematology, increased plasma oestrogen, and histopathology following castration.
Treatment
Castration with a wide margin of spermatic cord is the treatment of choice. For the patient with bone marrow suppression, perioperative supportive care such as fluid therapy, blood transfusions, platelet-rich plasma and broad-spectrum bactericidal antibiotics is indicated. Corticosteroids, anabolic steroids and haematinics have unknown therapeutic benefits. Haematopoietic growth factors such as granulocyte-colony stimulating factor (G-CSF) and erythropoietin may be tried.
Prognosis
Complete surgical resection is curative if there is no bone marrow hypoplasia or metastasis. Mortality rate is more than 70% with severe bone marrow depression (Sherding et al 1981). Haematological parameters can take months to normalize, despite removal of the source of oestrogen. However, the prognosis for patients with myelotoxicity is always guarded, and persistent severe thrombocytopenia of more than 2 weeks’ duration is a poor prognostic indicator. Dogs that survive have prolonged survival (sometimes more than 1 year) (Sherding et al 1981). Haemorrhage, depression, exercise intolerance and infections may occur with persistent pancytopenia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


