Oesophageal cancer in cats and dogs is very rare. The most common types are squamous cell carcinoma and other carcinomas, leiomyosarcoma, fibrosarcoma and osteosarcoma (Gualtieri et al 1999a, Jacobs & Rosen 2000, McCaw et al 1980, Ranen et al 2004a, Shinozuka et al 2001, Takiguchi et al 1997).
There are also reports of benign tumours, e.g. leiomyoma and plasmacytoma, especially in the terminal oesophagus and cardia (Culbertson et al 1983, Hamilton & Carpenter 1994, Rolfe et al 1994). Sarcomas secondary to Spirocerca lupi have been reported (Ranen et al 2004b). Most malignant oesophageal tumours are locally invasive and metastasize to draining lymph nodes and/or haematogenously. Secondary invasion of the oesophagus by other neoplasia can also occur, e.g. thyroid or heart base tumours.
Clinical signs
These include oesophageal disease (regurgitation, dysphagia, pain on swallowing, aspiration pneumonia).
Diagnostic work-up
Thoracic radiographs may show soft tissue opacity in the thorax, an abnormal gas shadow in the oesophagus, or signs of aspiration pneumonia. A positive contrast study may show narrowing of the oesophageal lumen; however, the clinician should take extreme care that no contrast agent is aspirated into the lungs.
Due to the risk of aspirating contrast agent, it may be preferable to diagnose the presence of an oesophageal mass with endoscopy, which also provides an opportunity for endoscopic biopsies to be taken. If the tumour is a leiomyoma or leiomyosarcoma (arising from the smooth muscle of the oesophageal wall), and it has not perforated/ulcerated through the submucosa into the oesophageal lumen, retrieval of a diagnostic biopsy specimen using endoscopy may be difficult. However, the visualization of a non-ulcerated, submucosal, well-circumscribed spherical mass is suspicious of a leiomyoma or leiomyosarcoma. An ulcerated mass may be more amenable to diagnosis via endoscopic biopsy.
If endoscopic biopsies are unhelpful, the surgeon may then choose to attain a larger sample of tumour for definitive histopathological diagnosis via a thoracotomy. An attempt at curative resection/excisional biopsy would then be appropriate if the tumour appeared excisable.
Treatment
The treatment of malignant oesophageal cancer is difficult at best. Contributing factors are poor exposure, lengthy resections, tension, difficulty in reconstruction of oesophageal deficits, and metastatic disease. Radiation is of limited value. The prognosis is generally very poor for malignant oesophageal cancer. The prognosis for benign smooth muscle tumours is excellent with curative surgical resection. In the rare diagnosis of oesophageal plasmacytoma or lymphoma, a good prognosis may also be expected. Solitary plasmacytomas may be completely excised; however, if this is not possible, chemotherapy is recommended, usually a combination of cyclophosphamide and prednisolone for a number of cycles until no tumour is visible on endoscopy.
If more than 3–5 cm (more than 20%) of oesophagus is removed, there is an increased chance of dehiscence due to tension (Hedlund 1997). Partial circumferential tears are less likely to cause stricture formation than complete circumferential tears. Repair can be augmented with muscle and omental flaps. Few papers have been written on oesophageal replacement for large defects. Successful replacement of cervical oesophageal defects with microvascular anastomosis of free autogenous jejunal grafts has been reported in 20 dogs (Bouayad et al 1993), and in one other dog (Gregory et al 1988). However, only 4 of 20 dogs survived when the jejunal graft replaced the thoracic oesophagus, mostly due to leakage into the thoracic cavity (Bouayad et al 1993).
Free autogenous colon grafts used to replace thoracic oesophagus failed in five of five dogs, but were successful in three of three dogs when used to replace part of the cervical oesophagus (Kuzma et al 1989). A technique of substituting thoracic oesophagus with a vascularized, tubed transversus abdominus muscle graft had a high risk of complication and mortality, and was proven unsuitable (Straw et al 1987). In a recent paper, four of seven dogs survived reconstruction of partial circumferential defects of the thoracic oesophagus with pedicled diaphragm flaps (Paulo et al 2007).
Gastric tumours
Canine gastric tumours
Tumours of the stomach are uncommon, and account for <1% of all tumours encountered in the dog. Average age is 8 years and males are more commonly affected with gastric adenocarcinoma and lymphoma (Couto et al 1989, Priester & McKay 1980, Sautter & Hanlon 1975).
Adenocarcinoma accounts for 70–80% (Swann & Holt 2002), and is more common in Belgian Shepherds, Collies and Staffordshire Bull Terriers (Gualtieri et al 1999b, Sullivan et al 1987). French Bulldogs are predisposed to gastric adenomas, which can cause gastric outflow obstruction (Neiger 2003). Adenocarcinomas are often scirrhous (firm and white serosally) and appear leathery.
Other tumours are leiomyosarcoma (second most common tumour) (Kapatkin et al 1992, Swann & Holt 2002), leiomyoma (Kerpsack & Birchard 1994) and lymphoma (Couto et al 1989). Rare stomach tumours include extramedullary plasmacytoma (Brunnert et al 1992), mast cell tumour (Ozaki et al 2002), fibrosarcoma, adenomatous polyp, squamous cell carcinoma, fibroma, and carcinoid tumours of the enterochromaffin cells (Neiger 2003).
In one study (Swann & Holt 2002), 74% of dogs with gastric adenocarcinomas had metastasis. Gastric adenocarcinomas occur most commonly at the lesser curvature and antrum (Withrow 2007). Leiomyomas often occur at the cardia, and grow into the lumen as a smooth, well-circumscribed mass (Kerpsack & Birchard 1994). Gastric lymphoma as an isolated tumour is rare in the dog.
Feline gastric tumours
Feline lymphoma is the most common gastric tumour in the cat. Affected cats tend to be younger and are often feline leukaemia virus (FeLV)-negative. Gastric lymphoma can be localized or diffusely infiltrative. Other tumours are rare in cats.
Clinical signs
These include progressive intermittent vomiting (often haematemesis), weight loss and inappetence/anorexia.
Diagnostic work-up
• Radiography
• Ultrasonography
• Endoscopy
• Routine blood tests
Radiography
Positive contrast radiographic studies may show a filling defect or an outflow obstruction.
Ultrasound
Ultrasound of the stomach wall has been shown to be a useful, sensitive tool to detect tumour (Lamb & Grierson 1999, Rivers et al 1997) and has the advantage of showing disruption to the layers of the gastric wall (infiltrative vs. non-infiltrative tumours) and is usually the diagnostic tool of choice. Ultrasound may also be useful for staging the disease (e.g. evidence of lymphadenomegaly, or metastasis to other abdominal organs). However, these images do not provide definitive histopathological confirmation of metastatic disease, and should be interpreted with caution. For example, liver and splenic nodules may be benign nodular hyperplasia and enlarged lymph nodes may be a reactive change. Ultrasound-guided fine needle aspirates of thickened stomach wall may be useful; however, endoscopic biopsies are generally preferable.
Endoscopy
Endoscopy (gastroscopy) is a very useful tool to diagnose the presence of tumour and for collection of biopsy samples. However, endoscopic biopsy samples are small and are not always diagnostic because of associated necrosis, inflammation and fibrosis, and surgical biopsy may be the only method of obtaining a reliable diagnosis.
Routine blood tests
Non-regenerative, normocytic, hypochromic anaemia may result from chronic blood loss. Vomiting can cause hypochloraemia, hypokalaemia and a metabolic alkalosis. Paraneoplastic hypoglycaemia may be seen with leiomyomas and leiomyosarcomas (Bagley et al 1996, Beaudry et al 1995, Bellah & Ginn 1996, Boari et al 1995).
Staging
Staging is undertaken using abdominal ultrasound (see above comments) and thoracic radiographs.
Treatment and prognosis
The treatment of choice for most gastric tumours is surgery. Treatment is complicated by stage of disease at presentation and difficult operative area, often with a debilitated patient. A wide partial gastrectomy should be performed if possible. A ‘Bilroth I’ (partial gastrectomy with preservation of the pancreas, pancreatic duct and bile duct) is much better tolerated than a ‘Bilroth II’ (partial gastrectomy, pancreatectomy and cholecystoduodenostomy). Pancreatic exocrine insufficiency, dumping syndrome and bacterial intestinal overgrowth are some of the complications associated with Bilroth II, causing significant patient morbidity (Ahmadu-Suka et al 1988). Smaller, more frequent feeds may be required with resection of >25% of the stomach wall.
Gastric lymphoma
Diffuse gastrointestinal lymphoma does not respond well to standard chemotherapy (Couto et al 1989), so if excision is possible, it is advised. In the authors’ opinion, if gastric lymphoma appears as a mass lesion rather than generalized thickening, it should be treated with surgical excision if possible, and if not, with debulking and adjuvant chemotherapy. A liver biopsy should be taken for staging purposes, and regional (mesenteric) lymph nodes should also be biopsied/removed if enlarged.
For dogs with isolated gastric lymphomas treated with complete excision with adjuvant chemotherapy, the prognosis may be better than had been previously thought. However, for those patients where the tumour cannot be excised or is not confined to the stomach, the prognosis is poor, even with combination chemotherapy (3–6 months) (Couto et al 1989).
Gastric adenocarcinomas
These tumours should be removed with 1–2 cm margins of grossly normal tissue if possible. Even with successful resection with clean margins, the prognosis for adenocarcinomas is poor, with most animals dead within 6 months (Elliott et al 1984, Olivieri et al 1984, Swann & Holt 2002). Chemotherapy and radiation therapy are not of any known benefit in animals with gastric adenocarcinomas. Anecdotally, response to gemcitabine has been reported. In theory, total gastrectomies can be performed (Sellon et al 1996); however, this is rarely seen as a viable option in veterinary medicine.
Gastric leiomyosarcomas
The median survival time for dogs with gastric leiomyosarcoma is better at approximately 1 year (Kapatkin et al 1992).
Gastric leiomyomas
Often discovered as an incidental finding during necropsy, surgery or endoscopy in the old dog, gastric leiomyomas can result in chronic vomiting and intermittent gastrointestinal (GIT) bleeding. They originate from smooth muscle layers of the stomach wall, can be single or multiple and often occur at the gastro-oesophageal junction. Typical lesions are mucosa-covered masses protruding into the lumen (Figure 15.1). Average age is 16 years, so they occur commonly in very old dogs, compared to 10 years for adenocarcinoma.
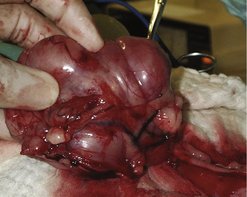 |
| Figure 15.1 (Courtesy R Straw.) |
Surgery is normally curative. As the tumour is benign and occurs in very old dogs, marginal excision is all that is needed. Full-thickness gastric wall resection is unnecessary and contraindicated due to increased risk and morbidity to the patient. The technique of choice is to incise through the stomach opposite the mass, and perform a submucosal resection (Beck & Simpson 1999).
Gastric extramedullary plasmacytomas
These are very rarely seen in the dog. Surgical resection with chemotherapy results in long-term survival (Brunnert et al 1992).
Gastrointestinal mast cell tumours
These tumours have a very poor prognosis with surgery (Ozaki et al 2002).
TUMOURS OF THE SMALL AND LARGE INTESTINES
Canine intestinal tumours
These are uncommon, accounting for approximately 3% of all tumours reported in dogs and 0.6% of all necropsies (Patnaik et al 1977). Intestinal tumours (small and large) account for 92% of all canine non-oral gastrointestinal tumours; 80% of dogs with small intestinal tumours are >7 years old (Gibbs & Pearson 1986).
Sex and breed predilection
Males may be over-represented with intestinal neoplasia (Birchard et al 1986, Couto et al 1989). German Shepherds and collies may be predisposed to non-lymphoid intestinal tumours (Birchard et al 1986, Gibbs & Pearson 1986, Patnaik et al 1977) and Boxers and Shar Peis predisposed to lymphoma (Coyle & Steinberg 2004).
Feline intestinal tumours
These account for approximately 4–9% of all tumours reported in cats. Intestinal tumours (small and large) account for 68–94% of all feline non-oral tumours (Brodey 1966, Cotchin 1959, Engle & Brodey 1969). Small intestinal tumours are more common in cats (Brodey 1966, Engle & Brodey 1969, Turk et al 1981).
Tumours of the small intestine
Clinical signs
Clinical signs include weight loss, inappetence, intermittent vomiting/diarrhoea, anorexia, bleeding (ulceration – anaemia, hypoproteinaemia, thrombocytopenia), peritonitis (abdominal pain, fever) and malabsorption (intestinal villi filled with neoplastic cells, leading to blocked lymphatics and obstruction).
Diagnostic work-up
Older animals show progressive weight loss and clinical signs of gastrointestinal disease. Anaemia is a common presenting sign characterized by hypochromasia and microcytosis. Hypoglycaemia in leiomyomas and leiomyosarcomas leads to weakness and seizures. In some cases an abdominal mass can be palpated (intestinal tumor or enlarged mesenteric lymph node), in other cases diffuse intestinal thickening with or without enlarged lymph nodes.
Radiography
Radiographs usually reveal an abdominal mass, intestinal accumulation of fluid/gas/ingesta, delayed transit time, mural lesions associated with filling defects, mucosal ulceration or displacement of adjacent bowel loops. Thoracic radiographs rarely demonstrate metastatic disease. Ascites maybe seen secondary to peritonitis.
Ultrasound
Ultrasound is more accurate than radiographs in identifying intestinal masses and at the same time allows evaluation of regional lymph nodes for metastatic disease (Figure 15.2). Ultrasound-guided fine needle aspiration (FNA) may be helpful (Bonfanti et al 2006), although histopathology is often needed for diagnosis.
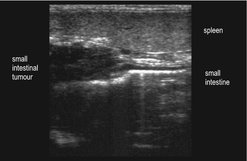 |
| Figure 15.2 |
Biopsy
Endoscopic biopsies are useful for proximal duodenal or rectal tumours, although these may not be diagnostic, even for lymphoma (Couto et al 1989). Laparotomy and biopsy for proximal and distal intestinal tumours is preferred as full-thickness intestinal biopsies are more likely to yield a definitive diagnosis (Kleinschmidt et al 2006).
Canine small intestinal tumours
Most canine small intestinal tumours are malignant.
Lymphoma
The most common intestinal tumour in dogs (and cats) is lymphoma; the majority are multifocal/diffuse and involve the small intestine (large intestine less common). Intestinal lymphoma makes up to 5–7% of all canine lymphomas (Madewell & Theilen 1987), and most are of T-cell origin (Coyle & Steinberg 2004, Miura et al 2004, Steinberg et al 1995). It has been postulated that lymphocytic–plasmacytic enteritis may be a precursor to intestinal lymphoma.
Isolated lesions are treated with surgical excision and adjuvant chemotherapy. Twenty-five per cent of dogs with gastrointestinal lymphoma have involvement of other organs (Chapter 22). Multiple intestinal lymphoma or metastatic disease is best treated by chemotherapy alone (unless there is obstruction or perforation). Diffuse canine alimentary lymphoma does not respond as well to chemotherapy as the multicentric form (Couto et al 1989), although others mention several cases of durable remission with CHOP (cyclophosphamide, vincristine, doxorubicin and prednisolone)-based protocols. The solitary form does better if it can be surgically removed, with or without adjuvant chemotherapy. Thirty cases of primary canine gastrointestinal lymphoma reported showed a poor prognosis when treated with surgery alone (n = 4), surgery and chemotherapy (n = 8), chemotherapy alone (n = 15) or supportive care alone (n = 3) with a median survival of 13 days, but longer survival for patients with disease of the large intestine (Frank et al 2007).
Adenocarcinoma
The second most common intestinal tumour in dogs (and cats) is adenocarcinoma (Figure 15.3), which is commonly annular and in the dog has been reported to have an increased incidence in the large intestine and rectum versus small intestine (Head et al 2002, Patnaik et al 1980). However, another paper reported 71% in small intestine and 29% in large intestine (Paoloni et al 2002).
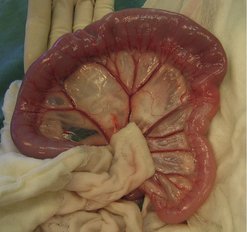 |
| Figure 15.3 |
There are four histological types: solid, acinar, mucinous and papillary (Patnaik et al 1980). They generally have similar biological behaviour, although tumours involving large segments of bowel may be slow growing, with horizontal spread and few metastases, i.e. papillary adenocarcinoma. Acinar, solid and mucinous types tend to show more vertical growth and extend into the wall and other organs.
In dogs, small intestinal adenocarcinomas have a guarded to poor long-term prognosis. They are usually quite advanced at time of diagnosis. Extension of the neoplasm beyond the bowel wall was found in 85% of dogs and 71% of cats at necropsy (Birchard et al 1986). Metastasis is frequently to the regional lymph nodes, especially mesenteric and iliac. Diffuse metastasis to peritoneal surfaces often causes ascites, (carcinomatosis), and carries a grave prognosis. Metastasis is also common to abdominal and thoracic viscera, especially liver, spleen, kidney, myocardium and lungs. The overall median survival time (MST) is 10 months if patients survive the immediate postoperative period. Overall survival time (ST) with metastasis at diagnosis is 3 months, and overall ST without metastasis at diagnosis is 15 months for non-lymphomatous small intestinal masses treated with surgical excision (Crawshaw et al 1998).
Another paper reported an MST of 233 days (for 6 large intestinal and 15 small intestinal adenocarcinomas) treated with surgical excision. Only gender appeared to influence survival. Female dogs lived a median of 28 days, whereas male dogs lived a median of 272 days (Paoloni et al 2002). The benefits of adjuvant chemotherapy are not known.
Leiomyosarcoma and leiomyoma
In dogs, leiomyosarcomas occur most frequently in the caecum and jejunum. The median age is 12 years. Leiomyosarcomas arise in the outer muscular layers, are nodular, locally invasive and may cause intestinal perforation, but are slow to metastasize (Bruecker & Withrow 1988). Metastasis is often to regional lymph nodes and liver. MST for leiomyosarcoma was 13 months for dogs that survived >2 weeks postoperatively (Kapatkin et al 1992). After excision, 1- and 2-year recurrence-free periods were 80.1% and 67.2% for small intestinal and 83.3% and 61.9% for caecal smooth muscle tumours (leiomyoma and leiomyosarcoma), respectively (Maas et al 2007). Cohen et al (2003) also reported long survival in patients with intestinal leiomyosarcomas (MST of 21.3 months); even dogs with documented metastases had good postoperative survival times (MST of 21.7 months).
Other canine small intestinal tumours
Fibrosarcoma, carcinoids, plasmacytomas and mast cell tumours are rarely reported in dogs.
Extramedullary plasmacytoma
Surgical excision ± melphalan and prednisolone warrant a fair prognosis.
Mast cell tumour (MCT)
MCT of the small intestine is rarely seen and in one published report the MST was 16 days (Takahashi et al 2000). In the authors’ experience, long-term survivors have been encountered when the tumour was solitary and no metastasis evident at the time of surgery.
Carcinoids
Found in the duodenum, ileum, colon and rectum, carcinoids arise from enterochromaffin cells in the mucosa. They contain high levels of serotonin and the release of biologically active amines results in ‘carcinoid syndrome’ (cutaneous flushing, abdominal pain, diarrhoea, dyspnoea). They metastasize to regional lymph nodes and liver.
Treatment
Optimal treatment is wide surgical resection and intestinal anastomosis for solitary malignant intestinal tumours. Margins of 4–8 cm of normal tissue should be taken either side of the mass. En bloc resection (tumour, mesentery and lymph node) may be considered and will give palliative relief even with metastatic disease present. Hepatic and pancreatoduodenal lymph nodes drain duodenum; jejunal lymph nodes drain the jejunum; the ileum is drained by colic and jejunal lymph nodes.
Complications include wound dehiscence resulting in peritonitis and stricture. The potential for tumour recurrence and metastasis depends upon the particular tumour type, as detailed above. Chemotherapy is indicated for diffuse and multicentric lymphoma, and perhaps for solitary intestinal lymphoma after surgery. For more discussion see Chapter 22.
Feline small intestinal tumours
Most feline small intestinal tumours are malignant. The most common is lymphoma, then adenocarcinoma, then mast cell tumour.
Lymphoma (also Chapter 22)
This is the most common feline gastrointestinal tumour. Mean age is 10–12 years. Most (86%) have a palpable abdominal mass (Carreras et al 2003, Mahoney et al 1995). Cats with intestinal lymphomas are usually FeLV-negative using serology, although the role of FeLV and feline immunodeficiency virus (FIV) is uncertain, as many are FeLV positive using polymerase chain reaction (PCR) and immunohistochemistry (IHC) (Jackson et al, 1993 and Jackson, Wood, Misra, Haines, 1996). FeLV and FIV status may be more important in Australian cats, with FIV strongly associated and FeLV weakly associated with feline intestinal lymphoma (Court et al 1997, Gabor et al 2001). FeLV status for intestinal lymphoma in cats was prognostic in one study for early stage disease (Mooney et al 1989), but was not prognostic in other studies (Jeglum et al 1987, Zwahlen et al 1998).
Inflammatory bowel disease may be a precursor to intestinal lymphoma in cats (Carreras et al 2003). The proportion of T- and B-cell phenotypes varies, with some reporting predominance of B cell, T cell or neither (Carreras et al 2003, Jackson et al 1996, Zwahlen et al 1998), although immunophenotype does not appear to be a prognostic factor in cats for intestinal lymphoma (Zwahlen et al 1998). In cats, gastrointestinal lymphoma is often part of multicentric disease (80% have multi-organ involvement) (Gabor et al 1998). Metastasis is often to regional lymph nodes, liver and kidneys. Alimentary lymphoma may be diffuse (one-third) or focal (two-thirds).
Treatment is chemotherapy, as surgery does not improve survival compared to chemotherapy alone (Zwahlen et al 1998). The role of surgery is mainly to relieve obstruction, and less commonly to obtain a definitive diagnosis or repair perforation and treat associated peritonitis.
The most significant prognostic indicator is initial response to chemotherapy, with cats that survive the initial induction period generally achieving long-term remission (Richter 2003). Cats that achieve a complete resolution of clinical signs do better (Carreras et al 2003, Malik et al 2001, Milner et al 2005, Zwahlen et al 1998). Patients with the small cell/lymphocytic form have a better prognosis than those with the lymphoblastic form (see below) (Fondacaro et al 1999).
Clinical stage, intestinal site or extent of intestinal involvement was not prognostic in one paper (Mahoney et al 1995). In a series of cats with lymphoma of various anatomical sites treated with chemotherapy, anatomical site, sex, age and clinical stage did not influence the duration of first response or survival time, but those that had a complete remission after initial treatment lived longest (MST of 654 days vs. 122 days) (Milner et al 2005). The overall median duration of first remission was 20 weeks, and overall MST was 40 weeks in 21 cats treated with combination chemotherapy (Zwahlen et al 1998). In 28 cats with alimentary lymphoma treated with chemotherapy, the median survival time was 50 days. However, about 30% of these cats achieved complete remission, ranging from 30 to 1700 days (median 213 days) (Mahoney et al 1995).
Subsets of feline intestinal lymphoma
• Lymphocytic/small cell: low grade
• Lymphoblastic: high grade
When treated with chemotherapy, small cell/lymphocytic intestinal lymphoma had a 69% complete remission rate for about 2 years compared to only 18% for <3 months’ duration for the lymphoblastic form. Lymphoblastic intestinal lymphoma was more likely to cause a palpable abdominal mass and require surgery for obstruction than lymphocytic (Fondacaro et al 1999).
Epitheliotropic intestinal lymphoma
This type of lymphoma is mostly T cell, and may be a continuum of feline inflammatory bowel disease. Five of nine cats with epitheliotropic intestinal lymphoma treated with chemotherapy were long-term responders, with an MST of 11 months; the other four responded poorly and were euthanized within 3.5 months (Carreras et al 2003).
Globule leucocyte tumour/large granular lymphocyte lymphoma
This form is characterized by large mononuclear cells with prominent azurophilic granules. Diffuse metastasis at diagnosis is common, and often rapidly progressive and fatal, although individual cases may do well with surgery (McPherron et al 1994).
Adenocarcinoma
This accounts for 20–35% of all feline gastrointestinal tumours (Cribb 1988) and 0.4–2.9% of all cat malignancies. Mean age is 11 years with Siamese cats predisposed. There is no association with FIV or FeLV. Adenocarcinoma tends to be found in small intestine, especially jejunum or ileum (Cribb 1988, Patnaik et al 1976). Histological subtypes include tubular, undifferentiated and mucinous (Turk et al 1981). Tubular may have a better prognosis (Cribb 1988). Most are advanced, with metastasis in 72% of cases at diagnosis (Kovosky et al 1988). Metastasis is to abdominal serosa (carcinomatosis), lymph node, liver and lungs.
Osteochondroid metaplasia has been reported (Turk et al 1981) and generally appears as firm, annular, constrictive masses; it is annular when the lumen is constricted 360 degrees and intraluminal when tumour spreads into the lumen as well as infiltrative into the wall.
Kosovsky et al (1988) reported on 23 cats with small intestinal adenocarcinoma that were treated with resection and anastomosis. Eleven died or were euthanized within 2 weeks, 12 survived an average of 15 months (1.5–30 months). Of these 12, 5 cats with known lymph node involvement survived a mean of 12 months, and 2 cats with omental carcinomatosis lived 4.5 and 28 months. In another study, the MST was 2.5 months when treated with resection and anastomosis (Cribb 1988). Cats given no treatment survived for 2 weeks (Birchard et al 1986, Kosovsky et al 1988).
Mast cell tumour (MCT)
This is the third most common feline gastrointestinal tumour after lymphoma and adenocarcinoma. Cats are more frequently affected than dogs. Mean age is 13 years. MCT more commonly involves the small intestine with equal distribution between duodenum, jejunum and ileum, and <15% colonic involvement. Peritoneal effusion is relatively common. Peripheral mastocytosis and eosinophilia are rare (unlike the splenic form). Peripheral eosinophilia has been reported in two cats (Bortnowski & Rosenthal 1992). Cytology of peritoneal effusion is often diagnostic. There is no association with ulceration (this is more likely in the systemic form).
Metastasis is common and sites include mesenteric lymph nodes and liver, and less commonly spleen and bone marrow. Histologically they are generally poorly differentiated with less prominent cytoplasmic granules. Prognosis is poor, as they do not respond to chemotherapy and most die soon after diagnosis (Howl & Petersen 1995). Anecdotally, solitary intestinal MCT without metastasis may have prolonged survival following end-to-end anastomosis.
Leiomyoma and leiomyosarcoma
These tumours are rare in cats.
Duodenal adenomatous polyps
Mean age is 12 years, 83% male castrated, Asian cats may be over-represented.
Clinical signs include acute and chronic vomiting with haematemesis, and anaemia has been reported in 50% of cases. Surgical resection and end-to-end anastomosis was curative in 15 of 18 cats that survived >2 weeks postoperatively (11 alive, 3 dead of unrelated causes and 1 lost to follow-up) (MacDonald et al 1993).
Carcinoids
Rare in the cat, carcinoids are occasionally seen in the duodenum or ileum. They arise from enterochromaffin cells in the mucosa. They contain high levels of serotonin and the release of biologically active amines results in ‘carcinoid syndrome’ (cutaneous flushing, abdominal pain, diarrhoea, dyspnoea). They metastasize to regional lymph nodes and liver.
Tumours of the large intestine
Clinical signs
These can include tenesmus, haematochezia, dyschezia, rectal bleeding not associated with defecation, rectal prolapse or increased frequency of defecation. Other signs include vomiting, diarrhoea and weight loss. Collapse and septic peritonitis with perforation is also possible. Debilitation and hypoproteinaemia may complicate treatment.
Diagnostic work-up
Abdominal palpation demonstrates a palpable abdominal mass and pain; rectal examination usually reveals a lesion/stricture. Proctoscopy will allow visualization of tumours and in many cases a biopsy can be obtained by proctoscopy.
An abdominal mass, abdominal effusion, filling defect, etc. may be seen using radiographs ± contrast study. Care should be taken if perforation is suspected as barium is very irritant (iodine is preferred). Thoracic radiographs rarely identify pulmonary metastasis.
Surgery provides the definitive diagnosis.
Canine large intestinal tumours
Adenomatous polyps
These are the most frequently reported tumours of the canine rectum (Figure 15.4) (Holt & Lucke 1985). Haematochezia was the most common (82%) presenting sign in 34 dogs with colorectal adenomatous polyps or carcinoma in situ (Withrow 1997). Most of these dogs presented with a solitary rectal mass. Recurrence of clinical signs after surgery was common (41%), and malignant transformation of the tumour was documented in 18% of cases. Local recurrence and malignant transformation occurred more often if multiple masses, diffuse disease or carcinoma in situ was initially diagnosed (Valerius et al 1997). Malignant transformation was also reported in three of five dogs, two of which suffered local recurrence at 16 and 24 months, and the third died of unrelated causes 5 months after surgery (Danova et al 2006), and in an earlier study in one of five dogs (White & Gorman 1987).
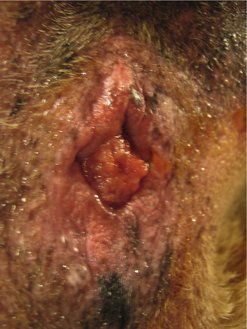 |
| Figure 15.4 |
Adenomatous polyps of the distal colon and rectum can be treated surgically by pedicle ligation or excision with clean margins, electrocautery, cryosurgery, transanal endoscopic treatment or piroxicam. Proctoscopy is ideal before surgical treatment as multiple polyps/masses may be present, and this will influence treatment and prognosis.
Medical management with oral or suppository piroxicam for 4–6 weeks showed a good to excellent response in seven of eight dogs with rectal polyps (Knottenbelt et al 2000). This treatment has the advantage of avoiding the need for surgery and its associated complications (e.g. dehiscence, peritonitis, stricture), although long-term follow-up and survival was not reported. Such palliative treatment may be an appropriate choice for many clients, but they should be aware that if tumour remains, there is the persistent potential for malignant transformation and, as a consequence, possible tumour-related morbidity or fatality.
Distal pedunculated lesions can be exteriorized and ligated at the base, avoiding a full-thickness rectal wall resection, but follow-up to monitor closely for recurrence (rectal palpation ± proctoscopy) is desirable, particularly if malignant transformation is detected histologically.
Full-thickness rectal wall resection of two distal rectal polyps (with histological characteristics of malignant transformation) with 1 cm ‘clean’ margins resulted in local recurrence after a prolonged period (16–24 months) and recurrent (malignant) disease was again treated surgically to result in further prolonged survival (14–86 months) (Danova et al 2006).
Holt & Lucke (1985) reported no recurrence in 14 of 15 dogs with rectal polyps treated with surgery (n = 14) or cryotherapy (n = 1). Four of five dogs with distal rectal polyps treated by exteriorization and resection with 1 cm margins (via anal approach) had long-term survival (17–84 months), the other was lost to follow-up (Danova et al 2006). Prolonged survival (1–5 years) with rare tumour-related deaths was also reported for 17 dogs treated surgically for rectal polyps (Seiler 1979).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


