CANINE TUMOURS
Oral tumours
Epidemiology
Tumours of the oral cavity of dogs account for approximately 6% of all tumours (Hoyt & Withrow 1984). They commonly arise from the gingiva but can arise from buccal mucosa, mandible, maxilla, palate, dental structures, tongue and tonsils.
A number of histological subtypes are recognized and as the prognosis and treatment options depend upon the histological diagnosis, early biopsies are recommended:
• Epithelial: squamous cell carcinoma (SCC), papilloma, fibropapilloma, intraosseous carcinoma and invasive nasal carcinoma
• Melanocytic: malignant melanoma (MM)
• Mesenchymal: fibrosarcoma (FSA), fibroma, rhabdomyosarcoma (rare), haemangiosarcoma (HSA), granular cell tumour (or myoblastoma), mixed mesenchymal sarcoma, neurofibrosarcoma, anaplastic sarcoma, myxosarcoma, chondrosarcoma (CSA), osteosarcoma (OSA), multilobular osteochondrosarcoma (MLO)
• Miscellaneous: transmissible venereal tumour (TVT), mast cell tumour (MCT), lymphoma (LSA), plasmacytoma.
For odontogenic tumours, the following tissues should be sampled:
• Epithelial: ameloblastoma, keratinizing ameloblastoma (cats only), ameloblastic adenomatoid, calcifying epithelial odontogenic tumour, hamartoma, dentinoma, odontoma, inductive fibroameloblastoma (cats only)
• Mesenchymal: fibromyxoma, cementoma, odontogenic fibroma
• Periodontal: acanthomatous epulis, ossifying epulis, fibromatous epulis.
Signalment
In general these are tumours of older dogs, except FSA seen in large breed dogs (e.g. German Shepherds, Golden Retrievers and Labrador Retrievers), papillary SCC seen in dogs of less than 6 months of age (Ogilvie et al 1988), undifferentiated malignancy of young dogs (less than 2 years) and viral-induced papillomatosis (less than 1 year). MM and FSA are more common in male dogs (Dorn et al 1968).
The breeds most commonly affected with oral tumours are Cocker Spaniels, Poodles, Weimaraner, German Shepherds, Golden Retrievers, German Shorthaired Pointers and Boxers (Cohen et al 1964, Dorn & Priester 1976, Todoroff & Brodey 1979). Small breeds are at a higher risk of MM and tonsillar carcinomas. Dogs with heavily pigmented oral mucosa are predisposed to MM (Dorn & Priester 1976, Dorn et al 1968).
Clinical signs
The clinical signs include decreased appetite, halitosis, loose/missing teeth, bloody saliva, exophthalmos, epistaxis, dysphagia, difficult/painful mastication and psychogenic polydipsia. Rostral tumours are most likely to by seen by the client and therefore diagnosed sooner than more caudally located tumours. Dyspnoea is common with large tonsillar, posterior pharyngeal tumours or lymphadenopathy.
Diagnostic work-up of the patient with an oral tumour
Imaging
Radiographs
Intraoral (best), open mouth, oblique lateral, dorsoventral (DV) and ventrodorsal (VD) to determine the extent of bone lysis but occasionally bone reaction is proliferative (e.g. OSA). The presence of no bony lysis on radiographs does not mean that there is no bony involvement. Radiographs underestimate the extent of bone destruction as lysis is only observed when more than 30–40% cortical bone loss has occurred. Fixation of tumour to bone suggests microscopic invasion of bone.
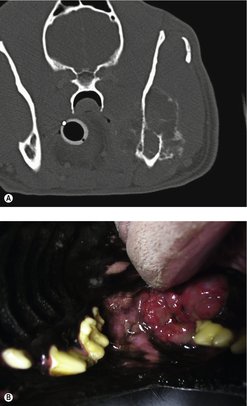
Computed tomography (CT)
The limitations of oral radiographs mean that CT is a much more sensitive diagnostic tool when considering the extent of local bone invasion prior to treatment and should be recommended when possible (Figure 13.1). Thoracic radiographs/CT are indicated for staging purposes.
Biopsy
• Large incisional (wedge) – at edge and centre of lesion
• Planned to minimize contamination of normal tissue
• Cytological preparation is usually unrewarding
• Cautery after biopsy may be required.
Routine blood work, haematology and biochemistry are indicated, plus urinalysis.
Staging (see Table 13.1)
• Staging is based on the size of the primary tumour, regional lymph node (RLN) involvement and distant metastasis.
• Fine needle aspiration (FNA) or excisional biopsy of the RLN is necessary if it is enlarged, fixed or painful.
• Thoracic radiographs or CT for staging.
| Stage | Characteristics |
|---|---|
| T – Primary tumour | |
| T0 | No evidence of tumour |
| Tis | Carcinoma in situ |
| T1 | <2 cm (1a = no bony invasion, 1b = bony invasion) |
| T2 | 2–4 cm (2a, 2b) |
| T3 | >4 cm (3a, 3b) |
| N – Regional lymph nodes | |
| N0 | No regional lymph node (RLN) involvement |
| N1 | Movable ipsilateral RLN (N1a = no tumour cells, N1b = tumour cells) |
| N2 | Movable contralateral or bilateral RLN (N2a, N2b) |
| N3 | Fixed RLN |
| M – Distant metastasis | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
Clinical staging grouping (see Table 13.2)
Most dogs have stage III or IV disease at diagnosis.
| The World Health Organization staging scheme for dogs with oral melanoma is based on size, with stage I =<2 cm diameter tumour, stage II = 2 to <4 cm diameter tumour, stage III => or = 4 cm tumour and/or lymph node metastasis, and stage IV = distant metastasis. | |
| Stage | Tumour, node, metastasis |
|---|---|
| I | T1 N0–2a M0 |
| II | T2 N0–2a M0 |
| III | T3 N0–2a M0 |
| IV | T1–3 N2b–3 M0, T1–3 N0–3 M1 |
Treatment
Surgery
The treatment of choice for the majority of oral tumours is surgical excision with margins. The margins required and the application of adjunctive therapy depend on histological diagnosis.
Radiotherapy
After surgery, radiotherapy is the most successful modality in the management of oral tumours in dogs.
• Radiation can be used as adjunctive therapy to clean up ‘dirty’ margins from incompletely resected MM, SCC, FSA, acanthomatous epulis, plasmacytoma, etc.
• Inoperable tumour of any histological type (palliative).
• As sole treatment for certain small tumours, e.g. plasmacytoma, acanthomatous epulis, MM and isolated LSA. Success of radiotherapy depends on histological type and clinical stage.
Chemotherapy
Chemotherapy has little application for oral tumours with the exception of LSA and plasmacytomas, but may have some role in the management of SCC in combination with the above modalities.
Malignant melanoma
This is the most common oral tumour in dogs and accounts for 30–40% of all oral malignancies (Bradley et al 1984, Dorn & Priester 1976, Todoroff & Brodey 1979). Typically seen in older dogs (mean age 12 years) (Ramos-Vara et al 2000) with possible sex predilection for males (Bjorling et al 1987, Dorn & Priester 1976, Todoroff & Brodey 1979). Dogs with heavily pigmented mucosa are at greater risk (Dorn & Priester 1976, Dorn et al 1968) and Chow Chow and Golden Retriever were over-represented in one study (Ramos-Vara et al 2000).
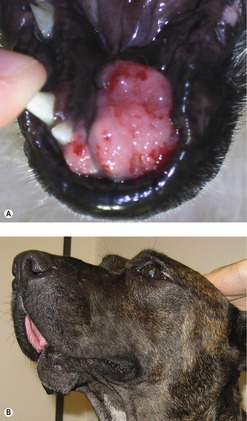
The site for these tumours is gingiva (42–63%), buccal or labial mucosa (15–33%), hard or soft palate (10–16%) and tongue (1.5–3.3%) (Dorn & Priester 1976, Todoroff & Brodey 1979). The tumour often appears firm and black (Figure 13.2) but 33% are unpigmented. It is not uncommon to have surface ulceration and necrosis. MM is rapidly growing and locally invasive.
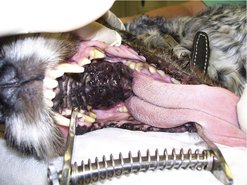 |
| Figure 13.2 |
Radiographic evidence of bone involvement is seen in 57% of patients at the time of diagnosis (Todoroff & Brodey 1979) and pulmonary metastases in 14% of cases. RLN metastasis has occurred in 53–74% of cases (Todoroff & Brodey 1979, Williams & Packer 2003). A RLN may be of normal size on palpation, but still be positive for metastasis (Williams & Packer 2003).
Amelanotic MM is the most probable tumour on any biopsy read as anaplastic or undifferentiated sarcoma or carcinoma. In such cases additional stains are required to confirm the origins of the tumour. Melan A (an immunohistochemical marker) was detected in 113/122 (92.6%) of oral MM (Ramos-Vara et al 2000).
Prognosis
Median survival times (MST) for dogs with oral melanoma treated with surgery are approximately 17–18, 5–6 and 3 months with stage I, II and III disease, respectively (Bergman 2007). Overall, 35% of patients with MM treated with mandibulectomy or maxillectomy will survive more than 1 year (Withrow & MacEwen 2001).
Prognostic indicators include:
• Size (<2 cm, MST was 511 days compared with 164 days if >2 cm or positive RLN) (MacEwen et al 1986). Smaller tumours are also more amenable to local control with radiotherapy (Blackwood & Dobson 1996, Théon et al 1997a).
• Ability of first treatment to give local control.
• Recurrence of MM after surgery negatively impacts on survival times.
• Location within the oropharynx may affect survival rates in that patients with tumours located on the lip or buccal mucosa develop metastasis late and are less likely to have positive lymph nodes at the time of presentation. Rostral mandibular sites also have a better prognosis than other locations (Hahn et al 1994, Kitchell et al 1994). Location influenced prognosis following multimodal therapy (curative intent surgery, palliative surgery, radiotherapy, chemotherapy, tumour vaccine therapy, gene therapy and immunotherapy), with MM of the lip surviving a median of 580 days, tongue 551 days, maxilla 319 days and hard palate 330 days. Small pedunculated melanomas on the gum or lip line are expected to behave better than larger intraoral MM (Withrow & MacEwen 2001).
• Histological criteria: Spangler & Kass (2006) described melanocytic oral neoplasms in 73 dogs; 92% of these were classified as malignant in the biopsy report, but malignant behaviour (i.e. metastasis or recurrence) was observed in only 59% of cases. A prognostic model based on nuclear atypia provided the most accurate (89%) prediction of overall behaviour. Another paper found a histological proliferation index (MIB-1) correlated with prognosis, as did lymphatic invasion for oral and cutaneous MM (Millanta et al 2002).
Indicators that are not prognostic: age, breed, sex, degree of pigmentation, microscopic appearance (with routine histological assessment) and DNA ploidy.
The prognosis is poor to fair, as 95% of dogs will eventually develop metastatic disease. With no treatment, the MST is 65 days (Harvey et al 1981). Metastatic rate is site, size and grade dependent.
Treatment
Surgery
The treatment of choice for oral MM is surgical excision with wide margins. This means that for patients with gingival tumours mandibulectomy or maxillectomy is indicated. A number of retrospective studies have evaluated the survival rates of dogs undergoing surgery alone. One recent study showed that in 78 dogs the MST was 20 months for dogs with stage I and II disease and 6 months for stage III and IV. This emphasizes the value of early detection and appropriate treatment. Earlier studies reported an MST of 9–11 months with a 12-month survival rate of 21% and a recurrence rate of 22% for dogs undergoing mandibulectomies. For maxillectomies the MST was 4.5–10 months, a 12-month survival rate of 27% and a recurrence rate of 48% (Kosovsky et al 1991, Schwarz et al 1991a).
The above illustrates the higher recurrence rate with maxillary tumours. Morbidity is greater with more extensive surgery. Client satisfaction with cosmetic and functional outcome is generally high (85%) (Fox et al 1997). Problems include dehiscence, recurrence and metastasis.
Prognosis with radiotherapy
MM responds well to radiotherapy (83–100% response rate with up to 70% having a complete response) (Bateman et al, 1994a and Bateman et al, 1994b, Blackwood & Dobson 1996, Freeman et al 2003, Théon et al 1997a). Typically a coarse fractionation protocol is used (3–4 fractions of 8–9 Gy).
The use of radiation in the management of oral MM depends on the size, location and goals of treatment for each patient. Proulx et al (2003) compared a number of radiation protocols and showed no significant difference in survival times based on protocol (9 Gy × 4 fractions, 10 Gy × 3 fractions, or 2–4 Gy × 12–19 fractions).
A number of studies have also looked to evaluate the efficacy of platinum-based chemotherapy in conjunction with radiation, but unfortunately results to date have shown no survival advantage of these combinations (Freeman et al 2003, Proulx et al 2003). For patients that have undergone surgery to reduce bulky disease down to microscopic disease, radiotherapy is used in the adjuvant setting. Using a total of 6 fractions (once weekly for 6 weeks or twice weekly for 3 weeks) the 1-year survival rate is 48%, 2-year survival rate 21% and the median disease-free interval (MDFI) 235 days/8 months and the MST 240–363 days (Freeman et al 2003). Palliation of MM (coarse fractions, 4 treatments) gave 69% complete response and 31% partial response (Blackwood & Dobson 1996). Metastasis was the major cause of death, and 58% of patients were euthanized or died of metastatic disease.
For patients with large tumours, where surgery is declined or when there is already evidence of metastatic disease, radiotherapy is a palliative treatment that can reduce tumour burden and improve quality of life. Typically the authors recommend three treatments once weekly when minimal disease is present and four treatments once weekly for palliative treatment. Positive lymph nodes should also be either irradiated or excised as part of a palliative protocol.
Prognosis with chemotherapy
MM is resistant to chemotherapy and there is no known effective chemotherapeutic agent for metastatic MM. A number of approaches using platinum-based drugs have attempted to improve long-term survival in patients with large primary tumours, e.g. intralesional cisplatin. In one study of 20 dogs, intralesional cisplatin gave 70% partial response with implant complications (mild local necrosis) in 85% of patients. MST was 51 weeks for responders (compared with 10 weeks for non-responders) (Kitchell et al 1994). Tumours located on the mandible had a better prognosis than maxillary tumours.
In another study on 27 dogs given carboplatin chemotherapy the response rate was 28%, with 4% complete response, 24% partial response and 36% each with stable disease and progressive disease. Median duration of partial response was 165 days; smaller dogs were more likely to respond but experienced a higher incidence of gastrointestinal side effects (Rassnick et al 2001).
It is in the field of immunotherapy that the next step forward is likely to occur with the development of a tumour vaccine.
MM immunotherapy
It is known that administering bacillus Calmette–Guérin (BCG) or levamisole does not improve survival. There is a mild survival advantage with advanced local disease with surgery and Corynebacterium parvum compared with surgery alone (MacEwen et al 1986). Intralesional injection of granulocyte–macrophage colony-stimulating factor (GM-CSF) or interleukin-2 (IL-2) induced partial or complete response and prolonged survival time compared to historical controls (Dow et al 1998).
Surgery and liposome muramyl tripeptide immunotherapy (L-MTP) showed that 80% of patients with stage I disease were still alive at 2 years compared with 25% who underwent surgery alone. L-MTP did not influence survival in patients with stage II or III disease (MacEwen et al 1986). IL-2 and interferon (IFN) induce natural killer (NK) cells, lymphokine-activated killer (LAK) cells and augment antibody-dependent cellular cytotoxicity, and are promising avenues of research because MM is highly immunogenic. Immunotherapy and radiotherapy may be synergistic.
Tumour vaccines
At the time of writing, a tumour vaccine has been given a conditional license by the US Food and Drug Administration for the treatment of canine patients with stage II–IV oral melanoma. The vaccine uses a DNA plasmid containing a human tyrosinase gene. Tyrosinase is present in canine melanoma cells and although similar to the canine tyrosinase the xenogeneic DNA stimulates an immune response against melanoma cells expressing this gene (Bergman et al 2006, Liao et al 2006). Currently the vaccine is recommended for patients that have received appropriate treatment, surgery or radiotherapy for local or regional disease that have an expected survival time of 60–150 days; those receiving the vaccine have been reported to have increased survival times up to 389 days (Bergman et al 2003).
Squamous cell carcinoma (SCC)
SCC accounts for 20–30% of all oral tumours in dogs (second most common after MM) (Cohen et al 1964, Dorn & Priester 1976, Kosovsky et al 1991). The mean age is 8–10 years. No sex or breed predilection has been identified but large breeds may be over-represented. Location within the mouth can be close to the incisors, premolars on the mandible or molars on the maxilla. The tongue and the tonsils are also sites of involvement.
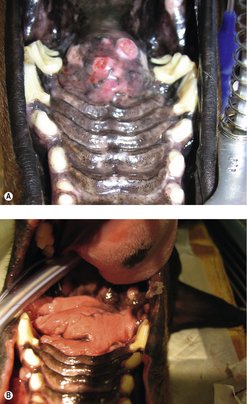
SCC present as irregular, raised, cauliflower-like, ulcerated masses (Figure 13.3A). At diagnosis 77% have radiographic evidence of bone involvement (Evans & Shofer 1988, Todoroff & Brody 1979). Evidence of RLN involvement is present in less than 10% of cases at diagnosis, although lymph nodes may be enlarged due to the production of inflammatory cytokines by the tumour. Pulmonary metastasis is present in a small percentage of patients at diagnosis. RLN and pulmonary metastasis are more common with caudal tongue and tonsillar SCC. Papillary SCC in young dogs is locally invasive but does not metastasize (Ogilvie et al 1988).
Prognostic factors
SCC of the base of the tongue and the tonsil is highly metastatic (metastasis in up to 73%) and recurs locally or regionally (Beck et al 1986, Brooks et al 1988). Good prognostic factors include rostral location for SCC originating from the gum. Long-term survival and cure is possible for SCC treated with appropriate surgery (not including tonsillar or base of the tongue).
Prognosis with surgery
The prognosis with surgery (particularly mandibulectomy) is good (Figure 13.3B). In one study, (Kosovsky et al 1991) the 12-month survival rate was 91% (MST 16–26 months), with only 10% of patients having recurrence. Good results were also reported after maxillectomy (Wallace et al 1992) with 12-month survival rates of 57% (MST 10–19 months) and a higher rate of recurrence (25%).
Prognosis with radiotherapy
For patients where radiotherapy is used only as a palliative option, without prior surgery, response rates were good to fair, with MST of 16 months. Radiotherapy can be used in the adjuvant setting when clean margins have not been achieved and the overall MST is the order of 34 months (LaDue-Miller et al 1996).
Prognosis with chemotherapy
There is no known effective chemotherapeutic agent for primary or metastatic SCC. Piroxicam has been advocated as a treatment option and in one study of 17 dogs resulted in 6% complete response, 11% partial response and 29% with stable disease. Location was not significantly related to response but median progression-free interval was significantly better for responders (180 days) than dogs with stable disease (102 days) (Schmidt et al 2001). Another study on nine dogs given cisplatin and piroxicam had unacceptable renal toxicity (Boria et al 2004).
Tonsillar squamous cell carcinoma (SCC)
Tonsillar SCC has been associated with pollution (10 times more common in urban areas) (Reif & Cohen 1971). Other tumours of the tonsil include LSA and melanoma (metastasis or primary lesion). Distant metastasis (lungs) is found in only 10–20% of cases at the time of presentation but the RLN are highly likely to be affected. Tonsillectomy is rarely curative and should be done bilaterally (due to a high percentage of bilateral disease, even if tonsils are not enlarged) (Todoroff & Brodey 1979).
Radiotherapy gives up to 75% control of local disease, although the prognosis is poor with a 12-month survival rate of less than 10% (Brooks et al 1988, MacMillan et al 1982). Chemotherapy is ineffective although cisplatin and bleomycin have been used with limited success (Brooks et al 1988, Buhles & Theilen 1973). Murphy et al (2006) reported five dogs with tonsillar carcinomas treated with surgical cytoreduction, followed by coarse fractionated radiotherapy together with carboplatin, with an MST of approximately 7 months.
The authors’ recommendation is tonsillectomy and radiotherapy (with one treatment at the time of surgery), followed by radiotherapy with carboplatin as a radiation sensitizer and follow-up carboplatin for 2–3 cycles. In a small number of cases MST is 10 months.
Lingual squamous cell carcinoma (SCC)
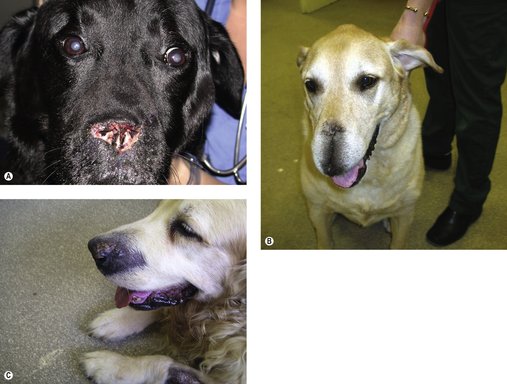
For SCC of the rostral tongue the prognosis with surgery (partial glossectomy) is fair. Up to 40–60% of the tongue (mobile portion) can be removed and is well tolerated. There may be postoperative dehydration and anorexia, and thermoregulation can be difficult in hot, humid conditions. In the initial postoperative period, some animals may benefit from a feeding tube (e.g. oesophageal, naso-oesophageal) and supportive fluid therapy until able to maintain adequate hydration and nutrition. The MST is 18 months. When surgery is not possible, then radiotherapy as a palliative is indicated (Figure 13.4).
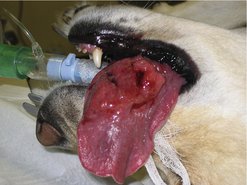 |
| Figure 13.4 |
Lingual SCC is graded I–III based on degree of differentiation, keratinization, mitotic rate, tissue invasion, vascular invasion, pleomorphism and scirrhous reaction. Prognosis depends on grade and stage of disease. Grade 1 MST is 16 months, Grade 2 MST is 4 months, and Grade 3 MST is 3 months (Carpenter et al 1993). Complete excision with 1 cm margins should be performed.
Prognosis with surgery or radiotherapy
The 12-month survival rate is 50% for complete resection and 60–80% for complete resection with low-grade histology (Carpenter et al 1993). Due to the difficult location, early detection is imperative to allow complete excision.
Radiotherapy can be used in the adjuvant or neoadjuvant setting. Side effects from radiotherapy include fibrosis of the tongue.
Fibrosarcoma (FSA)
This is the third most common oral tumour and accounts for 10–20% of oral tumours (Cohen et al 1964, Dorn & Priester 1976, Todoroff & Brodey 1979). The median age is 8 years and males may be predisposed. The most frequent location is the gingiva, usually on the maxillary arcade between canine and carnassial teeth, hard palate and buccal mucosa. Usually they are flat, firm, multilobulated and deeply attached. They are locally invasive to the gum and bone, and recurrence is common after surgical excision.
Radiographic evidence of bone involvement is seen in 60–65% of patients at the time of diagnosis (Todoroff & Brodey 1979); pulmonary metastasis is present in less than 10%. RLN metastasis is an infrequent finding at diagnosis. The common problem of all sarcomas is local recurrence and oral FSA presents an even greater challenge to the oncologist to establish local control.
Low-grade oral FSA with slow growth should be differentiated from low-grade oral FSA with aggressive biological behaviour (histologically low-grade yet biologically high-grade, or HLGBHG) (Ciekot et al 1994).
High-grade anaplastic oral FSA behaves similarly to HLGBHG with aggressive behaviour, and both have a higher metastatic potential than low-grade FSA with slow growth. Low-grade lesions with slow growth may be more likely to arise from the external surface of the maxilla, whereas HLGBHG occurred in the mandible in 28% of cases in one study (Ciekot et al 1994).
The cornerstone of treatment is surgery (mandibulectomy/maxillectomy). FSAs have a poor response to radiotherapy and chemotherapy (Thrall 1981). Radiotherapy can be used alone or in combination with surgical excision. Radiation in combination with surgery has been shown to be therapeutic. The response rates to chemotherapeutic agents are low and only of short duration (Withrow & MacEwen 2001). Local control is more important than metastatic disease for prognosis, as local recurrence is the most common cause of death.
Dogs with oral FSA treated with surgery alone had a local recurrence rate of 32–57%, with 1-year survival rates of 31–50%, MST of 9.5–11 months, and distant metastasis occurred in 27% (Schwarz et al 1991b, White 1991, Withrow & MacEwen 2001). A combined dorsolateral and intraoral approach for resection of maxillary tumours in 20 dogs achieved clean margins in 70% and a median time to recurrence of 24 months. This approach may prolong survival time for maxillary FSA compared to traditional intraoral techniques (Lascelles et al 2003). Dogs treated with radiotherapy alone achieved a median survival time of 7 months (Thrall 1981). For dogs with large tumours, preoperative radiotherapy to shrink the tumour prior to surgery should be considered.
Histologically low-grade/biologically high-grade (HLGBHG) oral fibrosarcoma (FSA)
HLGBHG oral FSA lesions are characterized by an innocent histological appearance but aggressive biological behaviour (Figure 13.5). They are reported to occur predominantly in the maxilla (72%) of large breed dogs, especially Golden Retrievers (54%) (Ciekot et al 1994). Biologically high-grade oral FSAs are often interpreted histologically as fibroma, low-grade FSA, nodular fasciitis, chronic inflammatory nodules or granulation tissue, due to fibroblast proliferation with minimal cellular aplasia, low mitotic rate, minimal nuclear pleomorphism and abundant production of collagenous matrix. However, histologically aggressive infiltration of surrounding muscle and bone and poor demarcation of adjacent normal tissue are also seen, despite low cellularity. Of 25 dogs, 72% had radiographic evidence of bone lysis (at presentation), 20% had regional lymph node metastasis and 12% had pulmonary metastasis at subsequent examination or necropsy (Ciekot et al 1994).
 |
| Figure 13.5 (Courtesy R Straw.) |
The prognosis is variable, and depends upon early diagnosis and aggressive treatment. Prolonged survival times can be achieved in some dogs with surgery, radiotherapy alone, surgery and radiotherapy, and radiotherapy and local hyperthermia (Ciekot et al 1994).
Oral undifferentiated malignancy of young dogs
This is a rapidly growing malignancy of young dogs (6–22 months), mostly found in large breeds. Histogenesis is undetermined on biopsies. The fast-growing mass in these dogs most commonly affects the hard palate, upper molar teeth, maxilla and orbit. Many dogs have metastasis. A biopsy shows an undifferentiated malignancy of undetermined histology. In one study, five of six dogs had metastasis beyond head and neck at post-mortem examination (Patnaik et al 1986). No effective treatment has been proposed. Euthanasia within 30 days of diagnosis is common, as these dogs have progressive debilitating disease (Withrow & MacEwen 2001).
Osteosarcoma (OSA)
OSAs account for 11.5% of canine oral tumours (Wallace et al 1992). Mandibular OSA accounts for approximately 27% of all axial OSA (and <4% of all OSA cases) (Heyman et al 1992). They are possibly associated with lower metastatic rate and a more favourable prognosis than appendicular OSA (see Chapter 21).
Multilobular osteochondrosarcoma (MLO)
The mean age for dogs with MLO is 7.5–8 years (Dernell et al 1998, Straw et al 1989). When treated with surgical resection, the MST is 800 days with less than 50% local recurrence and about a 50% metastatic rate (Figure 13.6). Adjuvant radiotherapy may be useful for incomplete resection (Straw et al 1989).
Histological grade is a prognostic indicator: Grade 1 MST is 50 months, Grade 2 MST is 22 months, and Grade 3 MST is 11 months (Straw et al 1989).
Lip and cheek tumours
The most common tumours are SCC, FSA, MCT, soft tissue sarcomas (STS) and MM. It is important to maintain the ability to open the mouth, therefore biopsy before planning definitive surgery, because radiation may be applicable before, after or instead of surgery.
Tongue tumours
Canine tongue tumours are uncommon with a possible predisposition in white dogs. SCC accounts for 50% of canine tongue tumours. Other differentials include granular cell myoblastoma, MM, MCT, FSA, adenocarcinoma (salivary duct or ectopic thyroid), HSA and haemangioma, rhabdomyoma and rhabdomyosarcoma. Non-neoplastic lesions include calcinosis circumscripta (cats and dogs) and eosinophilic granuloma (dogs).
The treatment of choice when possible is surgery. For patients not amenable to surgery radiotherapy may be beneficial (SCC, MM, MCT, HSA). Chemotherapy for tumours with known responsiveness may also be beneficial, e.g. MCT. More information on the treatment and prognosis for tongue tumours is discussed under the individual tumour types at the beginning of the chapter.
Odontogenic tumours
Epulides
Epulides are the most common benign oral tumours arising from the periodontal ligament. Fibromatous is the most common (57%), then ossifying (23%), acanthomatous (18%) and giant cell (2%) (Yoshida et al 1999). Another paper reported 40% acanthomatous, 32% ossifying and 25% fibromatous (Bjorling et al 1987). In this study, the median survival time post-surgery for all oral epulides was 49 months, with a 1-year survival rate of 92%.
Epulides – fibromatous and ossifying
Mean age is 8–9 years and they are seen more frequently in males (Yoshida et al 1999). They are often found on the maxillary premolars as pedunculated masses. Ossifying epulides tend to have a broader base of attachment and be less pedunculated than fibromatous types. They are non-ulcerative, non-invasive, covered by epithelium, do not invade bone and are grossly similar to gingival hyperplasia. Classification of fibromatous or ossifying depends on the histological presence or absence of bone.
Treatment is conservative surgical excision with or without electrocautery. Local tumour recurrence after conservative surgical resection (without bone removal) is 0–17% (Bjorling et al 1987, Bostock & White 1987).
Acanthomatous epulis – basal cell carcinoma
Mean age is 8 years, with no sex predilection and a reported breed predisposition in Shetland Sheepdogs (Yoshida et al 1999). The most common location is around the mandibular canines (60%) and incisors. They are locally invasive, with radiographic bone lysis in 80–90% at diagnosis, but do not metastasize. With wide surgery the prognosis is excellent (Bjorling et al 1987, White & Gorman 1989, Withrow & Holmberg 1983), with only 4% recurrence with appropriate surgery, a 12-month survival rate of 90% and MST of 36 months.
The treatment of choice is dependent on size, location and client preferences; however, wide surgical excision (extending 1 cm beyond the visible or radiographic margin of the tumour) is consistently curative. There is a high incidence of local recurrence after simple excision. Radiotherapy can be used as sole therapy for small lesions (Théon et al 1997b); however, if a complete response is not achieved, follow-up surgery is advised. The authors have used radiotherapy as a means of shrinking very large tumours, and then following with surgical resection.
Prognosis with radiotherapy is good with less than 5% recurrence rate and MST 37 months for small tumours (Thrall 1984). Malignant transformation has been reported in 5–20% of patients and older studies using orthovoltage machines reported bone necrosis in 6% of patients (Thrall 1984). Current day protocols use megavoltage radiation and control rates are good for small tumours; however, the treatment of choice is still surgery.
Intralesional chemotherapy (bleomycin) has been used once weekly for 3–10 treatments on four dogs with recurrent acanthomatous epulis. All (4/4) went into complete remission, with no tumour recurrence for a minimum of 1 year post treatment (Yoshida et al 1998). However, numbers were small.
Epithelial odontogenic tumours (Walsh et al 1987)
These tumours arise from dental lamina and may arise from either dental epithelium or nests of epithelial cells. Odontogenic tumours should be classified according to whether they are of epithelial, mesenchymal or mixed epithelial and mesenchymal origin, rather than based on inductive changes (Gardner 1992).
Inductive fibroameloblastoma
These rare tumours occur in young cats (6–18 months), more frequently in males. They are usually located rostrally, including the canine teeth of the mandible or maxilla (more common). There is a variable degree of bone destruction and proliferation; expansion with deformity of the teeth is common. The treatment is either mandibulectomy/maxillectomy or radiotherapy. Metastasis is not reported (Stebbins et al 1989).
Odontoma
Both rare and benign, these tumours arise from the dental follicle during early stage tooth development. The diagnosis is made when there is evidence of induction of both enamel and dentin. These are intraosseous and locally invasive but do not metastasize. Treatment includes surgical debulking and cryosurgery or wide surgical excision (Figueiredo et al 1974).
Non-inductive epithelial odontogenic tumours
Ameloblastoma and calcifying epithelial odontogenic tumour
Ameloblastoma is often confused with acanthomatous epulis on histology. Ameloblastoma is intraosseous and locally invasive but does not metastasize, and treatment is a wide surgical resection or surgical debulking and radiotherapy. MST following radiotherapy in dogs is 2 years (Langham et al 1977, Dubielzig & Thrall 1982).
Calcifying epithelial odontogenic tumour is a benign and rare tumour of the tooth-forming apparatus and produces a mineralized substance and amyloid. This causes a slow invasion of adjacent tissue resulting in osteolysis or deformation of mandible or maxilla. Treatment is either mandibulectomy or maxillectomy.
Oral tumour-like lesions
• Gingival hyperplasia
• Granulomatous reaction
• Osteomyelitis
• Peripheral giant cell granuloma
• Nasopharyngeal polyp
• Lymphocytic plasmacytic gingivitis-pharyngitis
• Odontogenic keratocyst
• Hamartoma
• Eosinophilic granuloma complex
Viral papillomatosis
The cause is horizontal transmission of papovavirus and usually occurs in young patients and more commonly in dogs (Norris et al 1985). The lesions are wart-like and multiple in the oral cavity, pharynx, tongue or lips. Spontaneous regression can occur in 4–8 weeks. Recommended treatment is surgery, cryosurgery or electrosurgery if severe or interfering with swallowing. Crushing lesions in situ may release antigen and result in immune-induced regression. The prognosis is excellent.
Canine oral eosinophilic granuloma
Seen in young dogs (1–7 years), there is a breed predisposition in Siberian Huskies and Cavalier King Charles Spaniels (Bredal et al 1996, Madewell et al 1980). These frequently raised, ulcerated lesions on the tongue often spontaneously regress, otherwise corticosteroids or surgical excision is required. Recurrence is rare.
Dentigerous cyst
These cysts arise within islands of odontogenic epithelium (Figure 13.7). They are benign, non-neoplastic circumscribed cystic lesions that may represent an early stage of a continuum to malignant epithelial tumours, e.g. basosquamous carcinoma (Poulet et al 1992). They are seen as closed cavities or cysts, with one or more teeth embedded in the cyst wall (Baxter 2004). Diagnosis is by radiographs with a characteristic radiolucent halo around a non-erupted tooth originating at the cemento-enamel junction and enveloping the crown of the tooth. Treatment of choice is surgical removal of the non-erupted tooth and cyst lining. Thorough curettage of the walls of the cyst is required to prevent recurrence.
 |
| Figure 13.7 |
Salivary gland tumours
These are rare in dogs. Mean age of affected dogs is greater than 10 years. No consistent breed predisposition has been reported (Hammer et al 2001). Mandibular and parotid glands are the most frequently involved (Carberry et al 1987). Although most are malignant (adenocarcinomas), benign lesions do occur (adenoma and lipoma) (Brown et al 1997, Stubbs et al 1996). Other malignant histological types include SCC, MCT, etc. Sialadenitis, sialoceles and salivary gland abscesses are differential diagnoses. Metastasis to RLN, lung, bone, eyes and kidneys has been reported (Grevel et al 1978, Habin & Else 1995).
Fine needle aspiration (FNA) cytology and biopsy are important for diagnosis (incisional biopsy prior to definitive surgery). The RLN should be palpated and assessed by FNA, or biopsy if enlarged. Thoracic radiographs/CT should be taken as part of staging. CT/MRI scans of local tumour may be of use to plan definitive surgical resection ± radiotherapy. Removal of the tumour with wide margins may be difficult due to location; however, a unilateral vagosympathetic trunk, jugular vein and carotid artery may be sacrificed with minimal morbidity in the dog. The surgeon should be familiar with normal regional anatomy of this area.
Radiation and chemotherapy may be used adjuvantly to surgery. This may improve survival times over surgery alone; however, detailed reports of survival outcomes comparing these groups are not available due to the small number of cases.
The authors suggest that dogs with non-metastatic disease have a better prognosis when treated with surgery than cats, as wider margins are easier to achieve in dogs. For large fixed tumours, radiotherapy can be utilized to shrink the tumour.
Histopathological features were not found to be prognostic in one study (Hammer et al 2001), although clinical stage was predictive.
Nasal tumours
Nasal planum tumours
These tumours are rare in dogs and by far the most common is SCC. SCC has been correlated with ultraviolet light and lack of protective pigment. Depending on the timing of the biopsy, SCC is often slowly progressive from carcinoma in situ to superficial SCC to deeply infiltrative SCC. Other cancers reported in this site are LSA, FSA, haemangioma, MM, MCT, fibroma and histiocytoma. Immune-mediated disease may present as erosive or crusty lesions on the nose but these are rarely proliferative, and usually other sites on the body are affected. Immune-mediated disease is probably not a contributing factor in tumour development.
History and clinical signs
Invasive SCC is usually preceded by a protracted course of disease (months to years) that progresses through stages. First there is crusting and erythema, then superficial erosions and ulcers (typically carcinoma in situ or early SCC) and, finally, deeply invasive and erosive lesions (Figure 13.8). Because SCC has a low rate of metastasis, which, if it occurs, does so late in the course of disease, dogs can have prolonged survival even if left untreated, although there is unacceptable morbidity associated with an ulcerated, invasive, deforming and secondarily infected cancer.
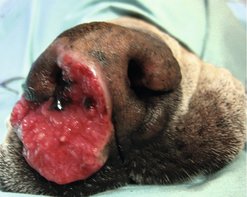 |
| Figure 13.8 |
Diagnostic work-up
Erosive or proliferative lesions should be biopsied (wedge). Care should be taken not to compromise a definitive surgical procedure (i.e. nasal planectomy).
Cytology and superficial biopsies are not useful. Lymph nodes are usually negative except with advanced disease and thoracic radiographs are invariably negative for metastases. Regional radiographs are generally unrewarding. CT/MRI are valuable for staging dogs with SCC of the nostril to help define the posterior extent of disease and hence level of resection. Rhinoscopy can also assist in determining the extent of caudal disease and to look for multiple lesions in dogs (Banks & Straw 2004).
Treatment
It may be possible to prevent or arrest the course of preneoplastic disease by limiting exposure to sun or tattooing to add pigment protection. Topical sunscreens are readily licked off and rarely help.
When inflammation and ulceration are present it is very difficult to maintain the tattoo as it is rapidly removed by macrophages. At best, tattoos would need to be repeated regularly.
Attempts to increase epithelial differentiation with synthetic derivatives of vitamin A are generally unsuccessful for advanced disease, but may be helpful in reversing or limiting the growth of preneoplastic lesions.
SCC can be divided into two categories depending on the degree of invasiveness:
1. Superficial, minimally invasive disease (can be treated effectively with cryosurgery, lasers, photodynamic therapy, intralesional carboplatin, hyperthermia or irradiation)
2. Deeply infiltrative disease.
In the authors’ experience, Golden Retrievers are over-represented with infiltrative SCC. If large, these tumours are difficult to control. The nasal planum should be completely removed with the tumour, if possible with margins of 1 cm of grossly normal tissue. If the margins are incomplete, adjuvant radiotherapy has been successful, resulting in a cure in one of four dogs (25%); however, the other three dogs had local recurrence at a median of 9 weeks (Lascelles et al 2000). Combined removal of premaxilla and nasal planum has been reported in the dog, with acceptable cosmetic appearance, good function and tumour control (Kirpensteijn et al 1994). An improved cosmetic appearance is reported with the use of bilateral labial mucocutaneous rotation-advancement flaps after nasal planectomy and premaxillectomy for SCC in the dog (Gallegos et al 2007). Another paper describes aggressive rostral maxillectomy and nasal planectomy. Complete margins were obtained in all cases, but one dog had local recurrence 10 months after surgery. All others had no signs of local recurrence during an 11–66 month follow-up period (Lascelles et al 2004) (Figure 13.9A,B).
Combined systemic chemotherapy and then surgery have been used in one dog to reduce tumour size, allowing it to be more amenable to wide surgical resection (Banks & Straw 2004); however, radiotherapy is more likely to be successful in the neoadjuvant setting. Surgical resection of the nasal planum with clean margins correlates to prolonged survival and potential cure (Gallegos et al 2007, Kirpensteijn et al 1994, Lascelles et al 2000). One potential problem in the months following surgery is stricture of the combined nasal orifice, and attempts at managing this in dogs include wide skin excision and removal of the nasal septum rostrally, laser ablation, rubber stents or permanent placement of a stainless steel intraluminal expansile stent.
Radiotherapy is applicable in the management of deeply infiltrative tumours as palliative therapy in patients where surgery is declined or when disease is very extensive; survival times range from 8 weeks to greater than 1 year. For early disease, excisional biopsy and radiotherapy is an option with good cosmesis and long-term control (Figure 13.9C).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree