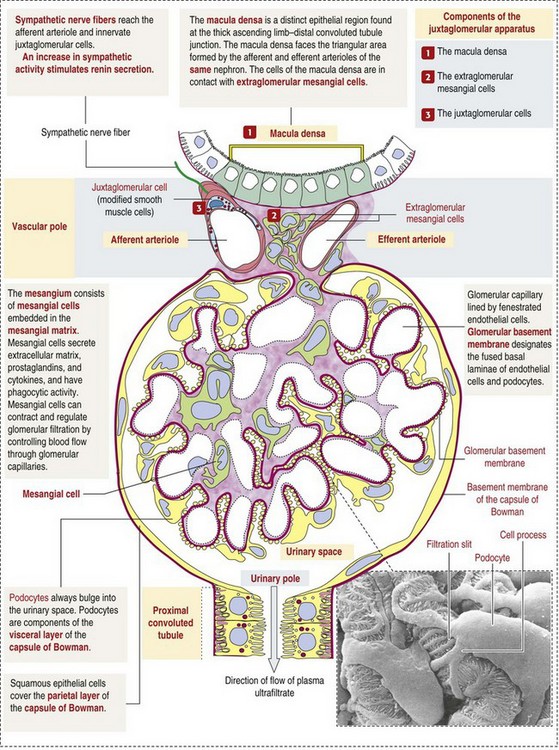
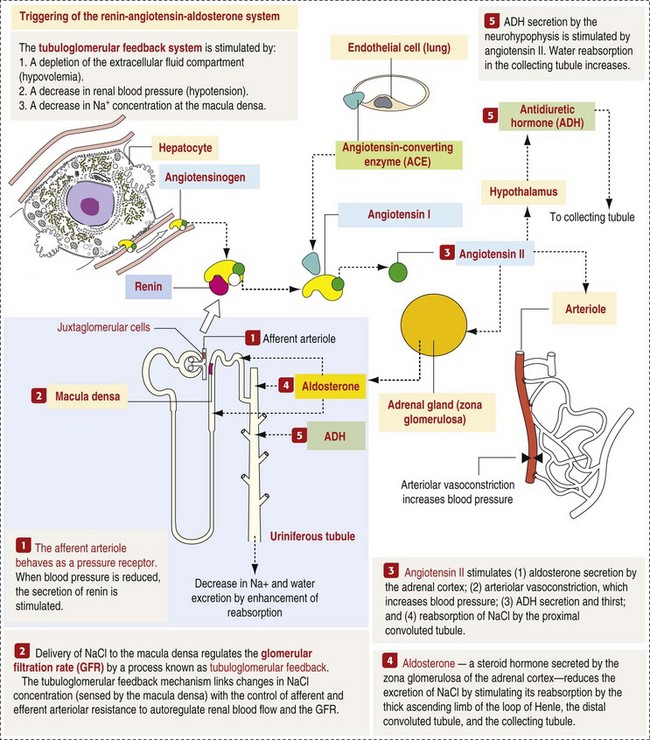
CHAPTER 11 Macroscopically, kidneys are organized functionally and anatomically into lobules. Each lobule represents collections of nephrons separated by the medullary rays (Fig. 11-1). Renal lobules should not be confused with renal lobes. Each lobe is represented by a renal pyramid (see Fig. 11-1). Among domestic animals, carnivores and horses have unilobar (or unipyramidal) kidneys. Porcine and bovine kidneys are multilobar (or multipyramidal), but only bovine kidneys have external lobation (Fig. 11-2). A diffuse fibrous capsule that in normal kidneys can be easily removed from the renal surface covers the kidneys. The renal parenchyma is divided into a cortex and medulla (Fig. 11-3). The corticomedullary ratio is usually approximately 1 : 2 or 1 : 3 in domestic animals. The ratio varies among species; for example, those adapted to the desert have a far larger medulla and thus a corticomedullary ratio that can approach 1 : 5. Normally the cortex is radially striated and dark red-brown except in mature cats, in which the cortex is often yellow because of the large lipid content of tubular epithelial cells. The renal medulla is pale gray and has either a single renal papilla, as in cats; a fused, crestlike papilla (renal medullary crest), as in dogs, sheep, and horses; or multiple renal papillae, as in pigs and cattle. The medulla generally can be subdivided into an outer zone, that portion of the medulla close to the cortex, and an inner zone, that portion closer to the pelvis. Papillae are surrounded by minor calyces that coalesce to form major calyces, which empty into the renal pelvis, where urine collects before entry into the ureters. Fig. 11-3 Schematic diagram, kidney. Microscopically, for ease of discussion, the kidney (and nephron) can be divided into four structural units: renal corpuscle (glomerulus and Bowman’s capsule), tubules, interstitium, and vasculature. The functional unit of the kidney is the nephron, which includes the renal corpuscle and renal tubules (the tubular system includes the proximal convoluted tubules, the loop of Henle, and the distal convoluted tubule). The uriniferous tubule is composed of the nephron and the collecting ducts, which are embryologically distinct from the renal tubules (Fig. 11-4). The uriniferous tubule is embedded structurally in the renal interstitium formed by a meshwork composed of stromal cells such as fibroblasts. The interstitium also contains the renal vasculature, which supplies blood first to the glomerulus and then to the renal tubules. Macroscopically, glomeruli are difficult to detect in the normal kidney but can be accentuated by lesions that allow them to be identified on cut section as randomly distributed granular foci or as red dots throughout the cortex. Microscopically, the glomerulus is a complex, convoluted tuft of fenestrated endothelial-lined capillaries held together by a supporting structure of cells in a glycoprotein matrix, the mesangium (Fig. 11-5). The entire glomerulus is supported by mesangial matrix that is secreted by the mesangial cells, a type of modified pericyte (Fig. 11-6). Mesangial cells are pluripotential mesenchymal cells, which are contractile and phagocytic and capable of synthesizing collagen and mesangial matrix, as well as secreting inflammatory mediators. The glomerular filtration barrier is composed of (1) pedicles of podocytes (visceral epithelium of Bowman’s capsule), (2) basal lamina (produced by both endothelial and epithelial cells), and (3) the fenestrated endothelium of glomerular capillaries (Fig. 11-7). Visceral epithelial cells (podocytes), aligned on the external surface of the basement membrane, are responsible for synthesis of basement membrane components and have special cytoplasmic processes (foot processes) that are embedded in the lamina rara externa. Negatively charged glycoproteins overlying the endothelial cells and the podocytes contribute to the charge differential of the glomerular basement membrane (GBM). Foot processes from adjacent visceral epithelium interdigitate to form filtration slits between them. Filtration slit diaphragms are composed of nephrin, a cell adhesion molecule of the immunoglobulin superfamily, which controls slit size by its connection to podocyte actin (see Fig. 11-7). The glomerular filtration barrier selectively filters molecules based on size (70,000 Da), electrical charge (the more cationic, the more permeable), and capillary pressure. In summary, both size-dependent and charge-dependent filtration is possible because of the porous structure of capillary walls, which is a function of endothelial fenestrations, a basement membrane formed of type IV collagen, basement membrane anionic glycoproteins, and filtration slits of the visceral epithelium. Bowman’s capsule is a cup-shaped sac at the beginning of the nephron that encloses the glomerulus (see Fig. 11-4). The renal tubular system (in the order of flow of urine) consists of a proximal tubule, loop of Henle, and distal tubule (see Fig. 11-4). The tubules connect to the renal pelvis at the distal end of the collecting ducts, and the whole structure, including the renal corpuscle, renal tubules, and collecting ducts is referred to as the uriniferous tubule (see Fig. 11-4). Macroscopically, the proximal and distal convoluted tubules are linked by the loop of Henle, which is divided into a descending and an ascending limb. The wall of the descending limb and initial portion of the ascending limb is thin (permeable), whereas the cortical portion of the ascending limb is thick (impermeable). Microscopically, the proximal tubule is lined by columnar epithelial cells that have a microvillous (brush) border. This arrangement greatly increases their absorptive surface, and their numerous intracellular mitochondria supply energy for the various secretory and absorptive functions. Distal tubules, collecting tubules, and the loop of Henle are lined by cuboidal epithelial cells that contribute to the concentration of urine by absorptive and secretory activities. Macroscopically, knowledge of the normal renal blood supply is important in understanding the pathogenesis and distribution of various renal lesions, especially renal infarcts. The kidneys receive blood primarily through the renal artery. An interlobar artery extends along the boundary of each renal lobe (renal column) and then branches at right angles to form an arcuate artery that runs along the corticomedullary junction (Fig. 11-8). Interlobular arteries branch from the arcuate artery and extend into the cortex. They have no anastomoses, making them susceptible to focal ischemic necrosis (infarct) as in any organ with end arteries. Microscopically, interlobular arteries have small branches that become afferent glomerular arterioles, which enter the renal corpuscle and subsequently exit at the vascular pole as efferent glomerular arterioles (see Fig. 11-8). Efferent arterioles supply the blood for the extensive network of capillaries that surround the cortical and medullary tubular system of the kidneys, known as the peritubular capillary network. The latter surrounds cortical segments of the tubules and then drains into the interlobular vein, arcuate vein, interlobar vein, and ultimately the renal vein. Additionally, the vasa recta are formed from the deeper portions of the peritubular network and descend into the medulla and around the lower portions of the loop of Henle before ascending to the cortex and emptying into venous vessels that connect to the interlobular and arcuate veins. The vasa recta parallel the descending and ascending limbs of the loop of Henle and the collecting ducts (see Fig. 11-4). Hence the blood supply to the tubules depends on passage through the glomerular vessels. The kidney has the following five basic functions: • Formation of urine for the purpose of elimination of metabolic wastes. • Acid-base regulation, predominantly through reclamation of bicarbonate from the glomerular filtrate. • Conservation of water through reabsorption by the proximal convoluted tubules, the countercurrent mechanism of the loop of Henle, antidiuretic hormone (ADH) activity in the distal tubules, and the urea gradient in the medulla. The tubular system is capable of absorbing up to 99% of the water in the glomerular filtrate. • Maintenance of normal extracellular potassium ion concentration through passive reabsorption in the proximal tubules and tubular secretion in the distal tubules under the influence of aldosterone. • Control of endocrine function through three hormonal axes: renin-angiotensin (see Fig. 11-8), most importantly, but also erythropoietin and vitamin D. Erythropoietin, produced in the kidneys in response to reduced oxygen tension, is released into the blood and stimulates bone marrow to produce erythrocytes. Vitamin D is converted in the kidneys to its most active form (1,25-dihydroxycholecalciferol [calcitriol]), which facilitates calcium absorption by the intestine. The GBM is structurally adept at separating substances based on size and charge. Additionally, the glomerulus is equipped with its own specialized mesangial cells, a component of the monocyte-macrophage system (see Figs. 11-6 and 11-7). Both size-dependent and charge-dependent filtration is possible because of the porous structure of capillary walls, which is a function of endothelial fenestrations, a basement membrane formed of type IV collagen, basement membrane anionic glycoproteins, and filtration slits of visceral epithelium. In addition to the principal glomerular function of plasma filtration, glomerular functions also include regulation of blood pressure by means of secreting vasopressor agents and/or hormones, regulation of peritubular blood flow, regulation of tubular metabolism, and removal of macromolecules from circulation by the glomerular mesangium. Integral to these functions is the juxtaglomerular apparatus, which functions in tubuloglomerular feedback by autoregulating renal blood flow and glomerular filtration rate. The juxtaglomerular apparatus is composed of four components: (1) an afferent arteriole whose smooth muscle is modified to form myoepithelial cells, which are the juxtaglomerular cells that secrete renin; (2) an efferent arteriole; (3) the macula densa; and (4) the extraglomerular mesangium. Renin, produced by cells of the juxtaglomerular apparatus, stimulates the production of angiotensin I from circulating angiotensinogen. The angiotensin-converting enzyme in the macula densa converts angiotensin I to angiotensin II, which then functions to constrict afferent renal arterioles; maintain renal blood pressure; stimulate aldosterone secretion from the adrenal gland, thus increasing sodium (Na+) reabsorption; and stimulate ADH release (Fig. 11-9). ADH principally increases the permeability of collecting tubules to water and increases the permeability of the medullary region to urea. A key function of the proximal tubules is to reabsorb Na+, chloride (Cl−), potassium (K+), albumin, glucose, water, and bicarbonate. This is facilitated by luminal brush border, basolateral infoldings, magnesium-dependent Na+ and K+ pumps, and transport proteins. The proximal tubule is continuous with the loop of Henle that is in close physiologic and anatomic association with the peritubular capillary network (within the cortex) and the vasa recta (within the medulla). The loop of Henle, via a countercurrent mechanism and Na+/K+-adenosine phosphatase (ATPase) pumps, absorbs Na+ and Cl− ions, producing a hypotonic filtrate that flows into the next portion of the nephron—the distal convoluted tubule. Here, water is reabsorbed from the tubule into the interstitium because of a solute concentration gradient and by the effects of ADH. The filtrate is further concentrated in the collecting ducts by water and sodium reabsorption by a Na+/K+-ATPase pump and additional water reabsorption into the medullary interstitium by a urea gradient. Intercalated cells of the collecting tubule regulate acid-base balance and reabsorb potassium. Thus the final excretory product, urine, is formed (Fig. 11-10). Acute Renal Failure: Acute renal failure can be caused by (1) tubular necrosis from infectious agents, such as bacteria (Leptospira spp., Escherichia coli, Streptococcus spp., Staphylococcus spp., and Proteus spp.) or viruses (infectious canine hepatitis virus, canine distemper virus, and canine herpesvirus); (2) obstructive nephropathy from urolithiasis, transitional cell neoplasms of the lower urinary system, or trauma; (3) renal ischemia with tubular necrosis from occlusive vasculitis/vasculopathy caused by bacteria, bacterial toxins, or tumor emboli; (4) tubular necrosis from nephrotoxic drugs, such as aminoglycoside-based antimicrobial drugs or antineoplastic drugs; and/or (5) tubular necrosis from chemicals, such as ethylene glycol and heavy metals. • Leakage of tubular ultrafiltrate from damaged tubules across disrupted basement membranes into the renal interstitium. • Intratubular obstruction resulting from sloughed necrotic epithelium. • Ascending disease, such as pyelonephritis • Intraluminal toxic metabolites derived from glomerular filtrate Fig. 11-11 Schematic diagram of ischemic renal failure. These alterations can occur after many insults, including the following: Azotemia and Uremia: Assays for plasma or serum concentrations of urea, creatinine, and the nitrogenous waste products of protein catabolism, are routinely used as indices of diminished renal function. The intravascular increase of these nitrogenous waste products is referred to as azotemia. Renal failure can result in the following: • Intravascular accumulation of other metabolic wastes such as guanidines, phenolic acids, and large molecular weight alcohols (example: myoinositol) • Reduced blood pH (metabolic acidosis) • Alterations in plasma ion concentrations, particularly potassium, calcium, and phosphate Chronic Renal Failure: Similar to acute renal failure, chronic renal failure occurs over a longer time duration and results in several additional hematologic and biochemical alterations. In the diseased kidney, production of erythropoietin, a stimulant of erythropoietic maturation, is reduced and contributes to nonregenerative anemia, as does uremia-associated increased erythrocytic fragility. Most animals in renal failure have hyperphosphatemia and low to normal calcium levels, although variations exist, depending on species and stage of the disease. Alterations in calcium-phosphorus metabolism in the uremic animal are a hallmark of chronic renal failure and result from a complex set of events as outlined in the following: • When the glomerular filtration rate is chronically reduced to less than 25% of normal, phosphorus is no longer adequately secreted by the kidneys and hyperphosphatemia results. • Because of the mass law interactions between serum calcium and phosphorus, ionized calcium concentration in serum is reduced as a result of precipitation of calcium and phosphorus. • Reduced ionized serum calcium stimulates parathyroid hormone (PTH) secretion, causing calcium release from the readily mobilizable calcium stores in the bone and from osteoclastic bone resorption. Renal secondary hyperparathyroidism is further thought to perpetuate and enhance renal disease by stimulating nephrocalcinosis (see Fig. 11-31; also see Chapter 12), the process by which renal tubular epithelium is damaged by an increase in intracellular calcium. Calcium is precipitated in mitochondria and in tubular basement membranes. Soft tissue calcification associated with uremia occurs in numerous sites and represents both dystrophic and metastatic calcification. These lesions are discussed in greater detail in the section on Disorders of Domestic Animals. The urinary system and especially the kidney can be exposed to injurious stimuli and agents via a number of routes (Box 11-1), including the following: • Localization within large renal vasculature. Massive infarction of the kidney is the result of large-bore renal vessel disease. • Localization within corticomedullary vessels. In the case of associated bacterial spread, septic embolic nephritis can occur. In those examples in which the embolus is nonseptic, the result is infarctive necrosis. • Localization within glomerular tufts. In this example, the lesions are localized to the vasculature of the small vessels within the glomerular tuft. • Localization within interstitial vessels. In this example, the lesions are localized to necrosis of interstitial tissues and tubules. Defense mechanisms unique to the renal system have evolved to counteract the routes of typical exposure to injurious agents and include those localized to the renal corpuscle, tubules, interstitium, and vasculature (Box 11-2). The most important of these barrier systems is the glomerular filtration membrane (see Fig. 11-7). The glomerular membrane is structurally adept at separating substances based on size and charge. Both size-dependent and charge-dependent filtration is possible because of the porous structure of capillary walls, which is a function of endothelial fenestrations, a basement membrane formed of type IV collagen, basement membrane anionic glycoproteins, and filtration slits of visceral epithelium. This inherent function of the glomerulus can also protect other regions of the nephron from damage by circulating inflammatory cells and their cytokines, as well as infectious agents that are present in the systemic circulation (i.e., bacteria in bacteremia). The response of the urinary system to injury is the response of each of its components—kidney, ureter, bladder, and urethra—to injury. Additionally, components within the kidney, such as the renal corpuscle, tubules, interstitium, and vasculature, have their own unique responses to injury. Responses to injury are described sequentially in this section and are summarized in Box 11-3. • Recruitment and localization of inflammatory cells at the site. • Release of inflammatory mediators and enzymes. • Destruction of glomerular structures such as the basement membrane. • Further compromise of nephron function. • Continuing damage by altered transglomerular hyperfiltration and perfusion shifts between nephron populations, so the less affected become overworked and succumb to the same fate.
The Urinary System*
Structure of the Kidney

A, Dorsal section through hilus, pig. B, Transverse section through hilus, dog. (Based on Schaller O, Constantinescu GM, eds: Illustrated veterinary anatomical nomenclature, Stuttgart, Germany, 2007, Enke Verlag.)
Glomerulus (Glomerular Tufts)
Glomerular Filtration Barrier
Visceral Epithelium (Podocytes)
Bowman’s Capsule
Tubules
Vasculature
Function of the Kidney
Function of the Glomerular Basement Membrane
Function of the Proximal Tubules
Renal Failure (Loss of Function)

A wide spectrum of clinical conditions can result in a generalized or localized reduction in renal blood flow, thus increasing the likelihood of ischemic acute renal failure. The most common condition leading to ischemic acute renal failure is severe and sustained prerenal azotemia. Kidney ischemia and acute renal failure are often the result of a combination of factors. (Redrawn from Thadhani R, Pascual M, Bonventre JV: N Engl J Med 334(22):1448-1460, 1996.)
Portals of Entry
Vasculature
Defense Mechanisms
Renal Corpuscle
Responses to Injury
Responses of the Kidney to Injury
Renal Corpuscle
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The Urinary System