Chapter 28 The Genera Campylobacter, Helicobacter, and Arcobacter
CAMPYLOBACTER
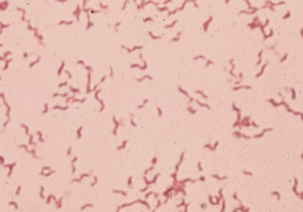
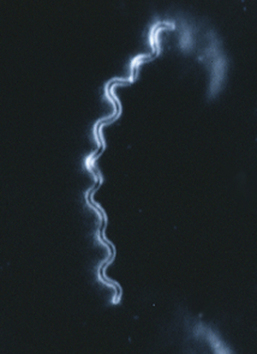
Members of the genus Campylobacter are gram-negative, slender, spiral to curved rods (Figures 28-1 and 28-2). Many were formerly in the genus Vibrio, leading to continuing references to the diseases they cause as vibriosis. Campylobacter spp. are motile, with a characteristic darting or corkscrew motion, best seen by darkfield or phase contrast microscopy. They are microaerophilic and require 3% to 5% CO2 and 3% to 15% O2 for growth. They are distinguished from true Vibrio species by their lack of oxidation and fermentation of carbohydrates. All are oxidase positive, some produce catalase, and most reduce nitrate. Urease negativity is common, and this trait is useful in distinguishing them from many members of the genus Helicobacter.
Campylobacter spp. frequently live as commensals in the intestinal tracts of mammals and birds. Campylobacter jejuni, the leading cause of bacterial food-borne illness, and Campylobacter coli, which contributes a minor share of cases, colonize food animals without producing illness. Non-jejuni and non-coli species have a role in animal disease. Animal-derived foods, especially those from poultry, are the major source of Campylobacter spp. for infection of humans. Species encountered in veterinary medicine are presented in Table 28-1.
TABLE 28-1 Campylobacter Species Encountered in Veterinary Medicine
| Species | Comments |
|---|---|
| C. coli* | Porcine, poultry normal intestinal flora; rare mild porcine diarrhea |
| C. fetus ssp. fetus* | Ovine abortion; sporadic bovine abortion; ruminant normal intestinal flora |
| C. fetus ssp. venerealis | Venereally transmitted bovine abortion and infertility |
| C. helveticus | Feces of normal and diarrheic dogs and cats |
| C. hyointestinalis ssp. hyointestinalis | Porcine normal intestinal flora; once thought to cause porcine proliferative enteropathy |
| C. hyointestinalis ssp. lawsonii | Stomachs of pigs; unknown virulence |
| C. jejuni ssp. jejuni* | Diarrhea in young dogs, cats, pigs, calves, lambs, ferrets, mink; sporadic ruminant abortion; “avian vibrionic hepatitis” in chickens and ratites; normal intestinal flora in most birds, ruminants, dogs, cats, rabbits, primates |
| C. lari | Feces of healthy gulls, other birds, dogs |
| C. mucosalis | Porcine normal oral, intestinal flora |
| C. sputorum ssp. bubulus | Normal genital flora in cattle and sheep of both sexes; differentiate from C. fetus |
| C. sputorum ssp. fecalis | Sheep feces, bovine semen and vagina; questionable virulence |
| C. upsaliensis* | Feces of healthy and diarrheic dogs and from healthy cats |
Diseases, Epidemiology, And Pathogenesis
Campylobacter fetus.
Subspecies of C. fetus have been recognized causes of ovine and bovine abortion for decades and, before the mid-1970s, were the major organisms of interest in the genus. Sheep infected with Campylobacter fetus ssp. fetus, by ingestion of the organism in contaminated food or water, develop bacteremia. Infection is sporadic, but in at least some cases, deposition of C. fetus ssp. fetus in placenta initiates an inflammatory process that is followed by abortion, usually in the third trimester (Figure 28-3). Infection by Campylobacter fetus ssp. venerealis, known as bovine venereal campylobacteriosis, is transmitted from bulls to cows in the normal process of breeding or by way of artificial insemination. The organism can typically be recovered from the glans penis and distal urethra of infected bulls, and deposition in the female reproductive tract results in ascend ing colonization that extends to the fallopian tubes. The syndrome usually manifests as infertility, and abortion occurs in less than 10% of infected cows.
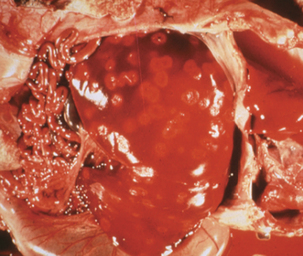
FIGURE 28-3 Ovine abortion resulting from Campylobacter fetus ssp. fetus.
(Courtesy J. Glenn Songer.)
Serum resistance probably plays a major role in virulence of C. fetus, and high-molecular-weight proteins composing the S (surface)-layer are the key components in this resistance. The S-layer is not effectively opsonized by complement component C3b, so neutrophil phagocytosis is limited. A 135-kD protein mediates resistance to phagocytosis by preventing binding of O-specific antibody. Mutants lacking the S-layer are serum sensitive and have reduced virulence in mice, suggesting that the S-layer masks Campylobacter lipopolysaccharide (LPS), perhaps in a bacterial strategy to decrease immunogenicity. S-layer proteins of C. fetus ssp. venerealis may be subject to antigenic shifts over time in experimentally infected heifers, and genetic analysis suggests that the basis for this phenomenon is in genomic rearrangements. The sapA promoter is on an invertible DNA fragment, which allows expression of two oppositely oriented S-layer gene cassettes, and recombination of 5′ conserved regions of multiple sapA homologs also contributes to antigenic variation. The S-layer is not required for adherence to epithelial cells in vitro.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree