Chapter 7 Surgical procedures for the conjunctiva and the nictitating membrane
Bulbar/palpebral conjunctival biopsy (punch/snip) 160
Surgical repair of conjunctival lacerations 161
Surgical repair of conjunctival defects 162
Adaptations in large animals and special species 162
Surgical treatment for symblepharon 162
Conjunctival grafts/transplantation 163
Substitute materials for conjunctival grafts 174
Adaptations in large animals and special species 174
SURGERIES OF THE NICTITANS 176
Surgical treatment of everted nictitans 176
Surgical treatment for hyperplastic lymphoid follicles 177
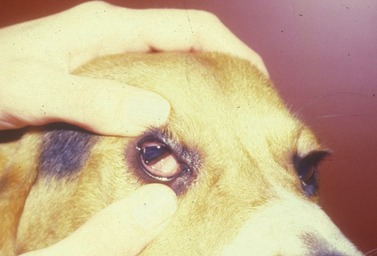
Surgical procedures for protrusion of the gland of the nictitating membrane or ‘cherry eye’ 178
Surgical procedures for prominent/protruded nictitans 184
Nictitating membrane flaps 185
Partial/complete excision of the nictitans 187
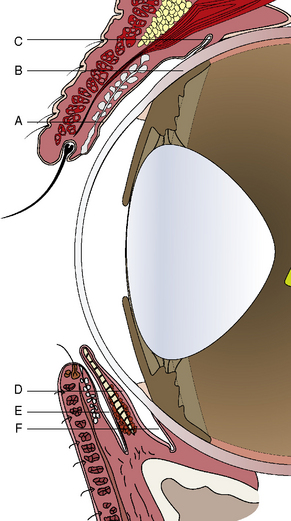
Conjunctival anatomy
Traditionally the conjunctiva is divided macroscopically into palpebral, fornix, and bulbar components. The palpebral conjunctiva lines the inner aspects of the eyelids. The bulbar conjunctiva mucosa starts at the corneal epithelial layer at the limbus. It covers Tenon’s capsule or bulbar fascia, and extends to join the palpebral conjunctiva at the fornix or the conjunctival cul-de-sac. Tenon’s capsule or bulbar fascia is quite thin in cats, horses, and cattle but variable in dogs, sometimes 5 mm or more thick. Medially a conjunctival apex or fold, the nictitating membrane or third eyelid, divides the ventral conjunctival fornix into two parts: the outer or palpebral portion, and the deeper or bulbar component (Fig. 7.1). As the conjunctival fornices span the globe and eyelids for 360°, the fornices may be divided into dorsal, lateral canthal, ventral, and medial canthal parts. The dorsal conjunctival fornix is deeper than the ventral fornix, and this may be necessary to accommodate downward eye movements. The ventral conjunctival fornix is shallower and its primary function is as a collecting basin for the tears. The medial conjunctival fornix is divided into anterior and posterior fornices of the nictitating membrane. Medial movement of tears toward the upper and, more importantly, the lower lacrimal puncta by the eyelid is not understood, but it appears to be an active rather than a passive process.
The palpebral conjunctiva originates at the eyelid margin (the margo-intermarginalis) and the orifices of the meibomian glands. The margo-intermarginalis is the last tissue that when contact occurs with the cornea and conjunctiva produces no damage; when the outer leading edge of the eyelid skin touches the cornea, as in entropion, corneal and conjunctival damage start! The palpebral conjunctival surface epithelium at the eyelid margin consists of non-keratinized stratified epithelium, but changes after several millimeters from the lid margin into pseudostratified columnar epithelium. Immediately beneath the palpebral conjunctiva is the thicker connective tissue, the tarsal layer, which contains the sebum-producing tarsal or meibomian glands. Once the pseudostratified epithelial surface is established, conjunctival goblet cells begin to appear and are most numerous in the fornices. These goblet cells produce mucin, an important deep component of the preocular or precorneal film, and an essential lubricant to prevent eyelid trauma to the conjunctival and the corneal surfaces (Fig. 7.2). Mucin forms the innermost layer of the preocular (precorneal) film and ranges in thickness from 1 μm on the cornea to 2 μm or more on the conjunctival surfaces. Mucin, a hydrated glycoprotein, forms an interface between the larger aqueous portion of the preocular film and the hydrophobic corneal epithelium. A relatively small portion of mucin is water soluble and part of the middle or aqueous fraction of the preocular film. The goblet cell-derived mucin decreases the surface tension of the preocular film, enhances the stability of the preocular film, and aids in the coherence of the aqueous portion of the preocular film to the corneal and conjunctival epithelia. Mucin also coats and reduces the irregularities of the corneal epithelium to produce an optically smooth corneal surface. The palpebral conjunctiva is quite transparent and often allows visualization of impacted or inflamed meibomian (or Meibom) glands on the deep aspects of both eyelids.
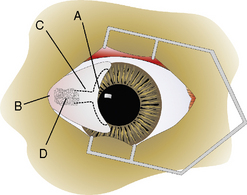
Anatomy of the nictitans
The gross anatomy of the nictitating membrane is quite similar among mammals. Located in the medial canthus, the nictitating membrane is a roughly triangular-shaped fold of conjunctiva, with the base of the triangle consisting of its free or leading margin (Fig. 7.3). Both anterior (palpebral) and posterior (bulbar) surfaces are confluent with the palpebral and bulbar conjunctival mucosa. Its free margin or border is usually pigmented in animals. When non-pigmented, the nictitans appears more prominent. Within the substance of the nictitating membrane is a hyaline T-shaped cartilage plate, which helps provide rigidity to the structure, assists conformation to the corneal curvature, and prevents disfigurement during movement (Fig. 7.4). The ‘arms’ of the T-shaped cartilage are immediately under its leading margin, and are relatively thin and slender compared to the thicker stem or base. The superficial gland of the nictitating membrane in both dogs and cats surrounds the base of the nictitans cartilage and produces seromucoid tears. Both dogs and cats possess a single nictitans gland, but in some species such as birds, the third eyelid gland may have two divisions. The deeper avian third eyelid gland is referred to as the Harderian gland.
Bulbar/palpebral conjunctival biopsy (punch/snip)
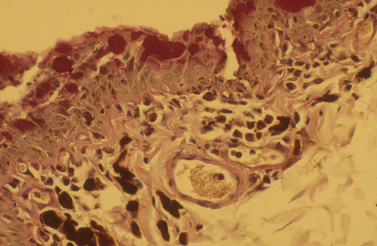
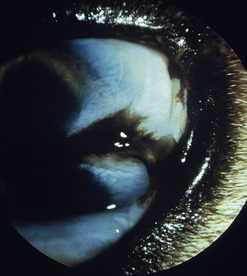
Biopsy of the conjunctiva may be indicated for diagnosis of non-specific diffuse and focal conjunctival inflammations (Fig. 7.5), and for possible neoplasia in all animal species. For biopsies of suspected conjunctival inflammations, selection of the ventral conjunctiva may be more rewarding. Focal swellings, such as conjunctival cysts, parasitic granulomas, nodular granulomatous episclerokeratitis, proliferative keratoconjunctivitis of Collies, and nodular fasciitis, can be biopsied or removed for histologic examination.
Surgical repair of conjunctival defects
Conjunctival defects may occur after the excision of large conjunctival dermoids (Fig. 7.6) and neoplasms, after loss from severe trauma, and extensive chemical burns. Fortunately, large conjunctival defects are infrequent in animals and may be repaired by a number of techniques. In contrast to the lid tumors in most species, conjunctival neoplasms tend to be more aggressive clinically and merit larger incisional margins during attempted excision. Small conjunctival defects (<1 cm) usually heal by secondary intention and resolve without adverse sequelae. Large palpebral and bulbar conjunctival defects (>1 cm) should be apposed by sutures. For bulbar conjunctival defects of about 2 cm or larger, the adjacent conjunctiva may be undermined and shifted to cover the defect. For larger defects, autografts of bulbar conjunctiva from the opposite fellow eye or the buccal mucosa may be transplanted. The mucosa is usually harvested free-hand, must be thin, and 1–3 mm larger than the defect to compensate for tissue shrinkage. The edges of the transplant are carefully apposed to the wound with 4-0 to 7-0 simple interrupted absorbable sutures. Often an incomplete temporary tarsorrhaphy is performed after conjunctival transplantation to prevent eyelid trauma and apply pressure to the surgical site to retard swelling. Autografts of conjunctiva and buccal mucosa to the conjunctiva in small animals are highly successful. When the conjunctival wound or autograft edges involve the limbal area, the conjunctival margin is apposed to the limbus by sutures to avoid overgrowth or migration onto the cornea.
Surgical treatment for symblepharon
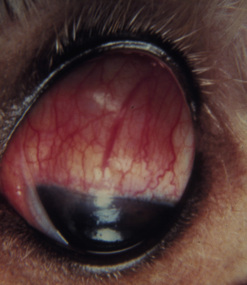
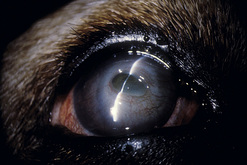
In dogs, symblepharon may develop after trauma, surgery, and chemical burns to the cornea and conjunctiva. In cats with ocular herpes (FHV-1), symblepharon may develop usually involving the cornea and the bulbar conjunctiva, the palpebral conjunctiva, or a combination of both conjunctivae (Fig. 7.7). Symblepharon associated with FHV-1 can be corrected successfully in cats, but since these corneal and conjunctival inflammations are often chronic, symblepharon formation may recur. Symblepharon affecting the cornea produces disfigurement and, if extensive, impairment of vision. Adhesions involving the bulbar and palpebral conjunctivae may shallow the conjunctival fornix, impair the drainage of tears, produce chronic conjunctivitis, and retard ocular motility.
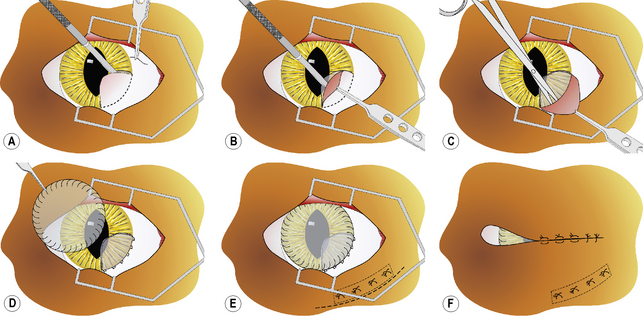
After general anesthesia, clipping of the eyelid hair, and surgical preparation of the eyelids, the area is draped for aseptic surgery. The conjunctiva is thoroughly cleaned with sterile saline and all foreign material removed by cotton-tipped applicators. After placement of a wire speculum to retract the eyelids, the conjunctiva adhered to the cornea is removed by superficial lamellar keratectomy. The periphery of the corneal lesion is incised by the Beaver No. 6400 microsurgical blade to the level of the superficial stroma (Fig. 7.8a). After lifting the edge of the incision with thumb forceps with 1 × 2 fine teeth jaws, the adherent conjunctiva is excised from the corneal surface (Fig. 7.8b). If the symblepharon continues into the conjunctiva, the incision is continued and the affected conjunctiva excised (Fig. 7.8c). Once the conjunctiva is freely moveable, its edge is apposed to the limbus with 5-0 to 7-0 simple interrupted absorbable sutures. If a defect remains in the bulbar and/or palpebral conjunctiva, its edges are apposed with 5-0 to 7-0 simple interrupted absorbable sutures. To cover the healing cornea and prevent the development of new adhesions between the cornea and conjunctiva, a plastic methyl methacrylate corneal protector (Crouch corneal protector; Storz, St Louis, MO) may be inserted or amniotic membrane apposed by sutures. A soft corneal contact lens may be used instead of the thicker corneal protector (Fig. 7.8d). If considerable adhesions are present between the bulbar and palpebral conjunctivae, a thin strip of silicone sheeting is fashioned to fill the area and secured in position with 4-0 to 7-0 simple interrupted non-absorbable sutures as well as 5-0 to 7-0 simple mattress sutures placed through the silicone strip and the full-thickness eyelid with the suture knots on the external lid surface (Fig. 7.8e). To retain the corneal contact lens and reduce eyelid movements, a partial temporary tarsorrhaphy is performed with 4-0 to 6-0 simple mattress sutures positioned at one-half thickness of the eyelids (Fig. 7.8f). For details on how to perform the temporary tarsorrhaphy, see Chapter 5. After recovery from general anesthesia, an E-collar is placed on the animal to prevent self-mutilation of the surgical site.
Conjunctival grafts/transplantation
Conjunctival autografts
Conjunctiva to conjunctiva
Conjunctival autografts are performed under general anesthesia and routine surgical preparation of the eyelids and conjunctival surfaces. Conjunctival grafts must be thin and devoid of most of the underlying connective tissues. Most conjunctival grafts are either free-hand island or pedicle types. Pedicle grafts are preferred if sufficient adjacent conjunctiva is available. Mucous membrane grafts should be free of pigmentation. The conjunctival graft site must be carefully prepared, and any necrotic or potentially infected tissues removed. The adjacent bulbar conjunctiva is incised by small tenotomy scissors to produce a pedicle flap to cover the surgical defect (Fig. 7.9a). The thin conjunctival pedicle should be 1–2 mm larger than the graft site to compensate for graft shrinkage. As the scissors undermine and separate the conjunctival mucosa from Tenon’s capsule, the scissors’ tips should be plainly visible when the graft is sufficiently thin. Once fitted to the graft site, the edges of the graft and conjunctival mucosa are carefully apposed to ensure epithelium to epithelium apposition with 5-0 to 7-0 simple interrupted absorbable sutures (Fig. 7.9b). A partial temporary tarsorrhaphy can be used to decrease eyelid trauma, and provide pressure to facilitate apposition of the graft to the underlying Tenon’s capsule.
Conjunctival autografts to cornea
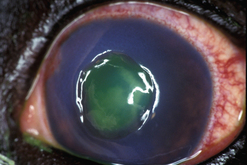
Conjunctival autografts are frequently used in small animal ophthalmology in clinical management of deep corneal ulcers, descemetoceles, and perforated corneal ulcers (Figs 7.10–7.12). Conjunctival autografts consist of either bulbar or palpebral conjunctival mucosa with epithelium and connective tissue (fibroblasts, blood vessels, and lymphatics). These autografts can be transposed and sutured onto the cornea to provide additional support and tissue for a cornea weakened by deep ulceration, descemetocele, or perforation with or without iris prolapse. The transplanted conjunctival autograft provides additional tissues and no risk of host rejection.
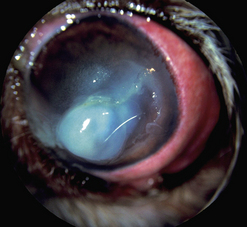
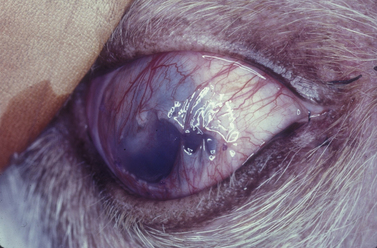
Fig. 7.10 Extensive keratomalacia in a young dog. This patient is a candidate for a conjunctival autograft.
Conjunctival autografts provide sufficient tissue to strengthen a weakened cornea and prevent staphyloma formation. If additional strength is indicated, a frozen section of sterile cornea or sclera is positioned in the corneal defect before the conjunctival graft is applied. Conjunctival grafts provide new and often highly viable epithelium. When harvested from the limbal area, the transplanted conjunctival epithelium is also a stem cell capable of additional generation and transition into corneal epithelium. The conjunctival autograft contains blood vessels and lymphatics to offer significant antibacterial, antifungal, antiviral, antiprotease, and anticollagenase effects. With conjunctival transplants, leukocytes, antibodies, serum, and α2-macroglobulin (thought to be the anticollagenase factor) are immediately incorporated into the corneal ulcer bed. Because of the conjunctival blood vessels, systemic antibiotics can enter the ulcer site in higher levels. The fibrovascular or deeper layer of the conjunctival transplant offers immediate fibroblasts and collagen to begin rebuilding the corneal stroma (Fig. 7.13).
Fig. 7.13 (a) Descemetocele in a dog treated by a pedicle conjunctival graft (6 weeks postoperative).
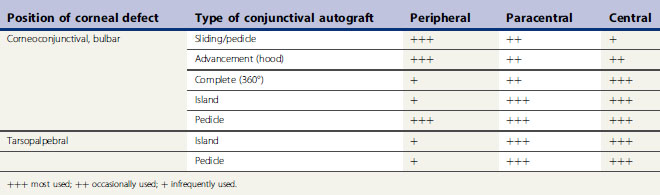
There are several different types of conjunctival autograft (Table 7.1). The divisions are based on the source of the mucosa (bulbar, tarsopalpebral, or corneoconjunctival) and the type of graft (advancement, bridge, complete, island (free), or pedicle). The dorsal bulbar conjunctiva is the most frequent source of mucosa, because of its accessibility and large surface area. The transpalpebral graft is usually constructed from the upper eyelid and sufficient tissue is available for any size corneal defect. Generally, the more central the corneal defect, the more critical the conjunctival grafting procedure.
The different types of conjunctival autograft have different clinical characteristics that influence their clinical use (Table 7.2). The larger the surface area of the cornea covered by the conjunctival graft, the greater the postoperative impairment to patient’s vision, the greater the barrier to postoperative intraocular examination, and, at least theoretically, the greater impediment for the corneal and intraocular penetration of most ophthalmic drugs. In those types of graft used to cover the central cornea, and for the more serious corneal ulcers, these techniques are often more difficult to perform. Attachment of the conjunctival graft to the progressive central corneal ulceration must be exact. Magnification provided by a head loupe or preferably the operating microscope is necessary for those types of conjunctival autograft that are apposed by sutures directly to the adjacent corneal epithelium and stroma.
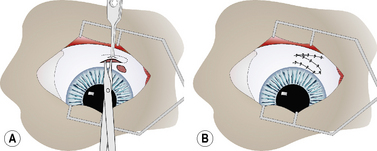
Complete (360°) bulbar conjunctival autograft (Gundersen type)
For the 360° fornix-based conjunctival graft, the dorsal bulbar conjunctiva is elevated by fine teeth thumb forceps and incised by scissors at the limbus (Fig. 7.14a). The bulbar conjunctiva is separated from the underlying Tenon’s capsule by alternating blunt–sharp dissection by small tenotomy scissors with blunt tips. For a reasonably thin conjunctival graft, the scissors’ tips should be easily observed through the thin mucosa (Fig. 7.14b). To facilitate dissection, saline can be injected subconjunctivally to help separate the bulbar conjunctiva from Tenon’s capsule. Some hemorrhage is expected and depends on the extent of conjunctival hyperemia associated with the corneal ulceration and secondary iridocyclitis. If the surgical dissection plane enters Tenon’s capsule, additional hemorrhage results.
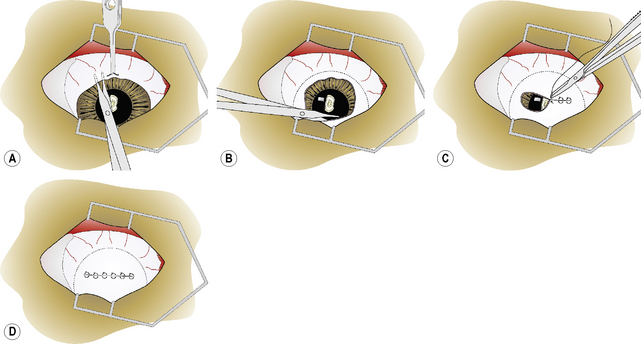
Fig. 7.14 In the 360°, or Gundersen-type, conjunctival graft,
(d) Once completed, the 360° bulbar conjunctival graft covers the entire cornea.
The bulbar conjunctiva is dissected for 360° about the limbus. The most difficult area is usually under the nictitating membrane (Fig. 7.14b). As the cornea measures about 15 × 16 mm in the dog, and 16 × 17 mm in the cat, in vertical and horizontal diameters, respectively, adequate amounts of bulbar conjunctiva necessitate 8–10 mm of dissection from the limbus for 360°. As the different rectus muscles in the dog and cat insert 6–10 mm from the limbus, preparation of the conjunctival graft requires surgical dissection immediately beneath the bulbar conjunctiva and not on the sclera.The conjunctival graft must be thin to minimize traction and excessive pressure on the sutures postoperatively. When properly prepared, the loosened edges of the bulbar conjunctival graft should rest on the central cornea and not retract spontaneously to the limbus.
The edges of the bulbar conjunctiva are apposed horizontally with 5-0 to 7-0 absorbable simple interrupted or simple mattress sutures (Fig. 7.14c). Usually four to six sutures are necessary to appose the dorsal and ventral conjunctival edges. Simple interrupted mattress sutures are recommended if the graft is thicker than desirable or additional traction on the suture line is anticipated. A purse-string stitch has also be used but is not recommended as this produces additional tension on the graft as all edges are pulled to the center of the cornea. Once completed, the 360° bulbar conjunctival graft covers the entire cornea (Fig. 7.14d).
The transposed conjunctival mucosa is not usually sutured directly to the corneal defect, but can be if perforation is likely or the deep corneal ulcer is already leaking aqueous humor (Fig. 7.15). As these grafts completely cover the cornea, vision in the eye is obscured and intraocular inspection is not possible.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree