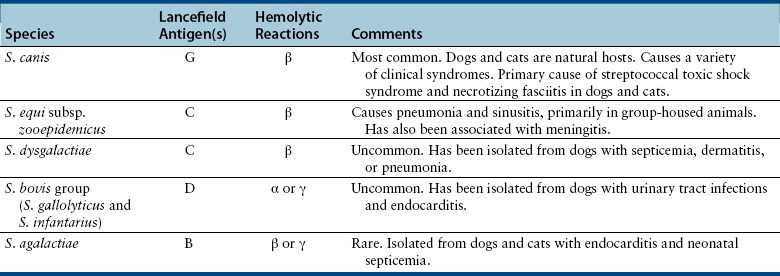
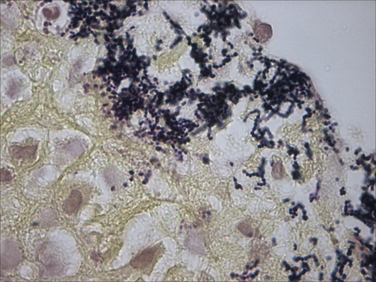
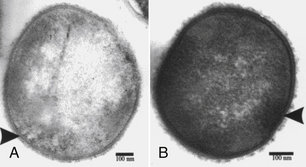
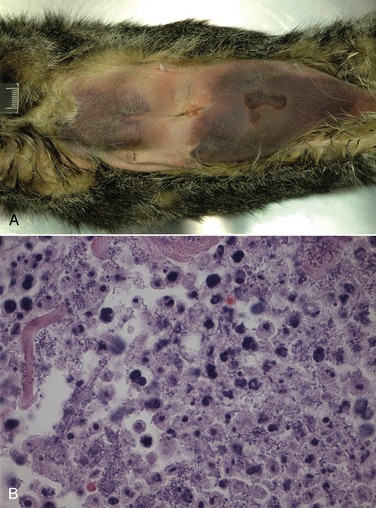
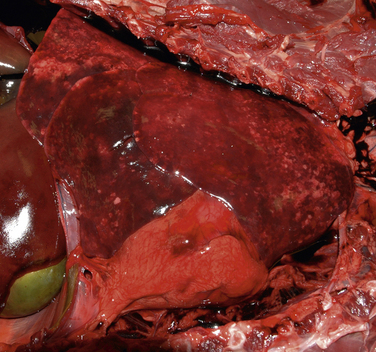
Chapter 34 Streptococci are catalase-negative, gram-positive cocci that divide along a single axis, forming pairs and chains of organisms (Figures 34-1 and 34-2). They are facultative to strict anaerobes that require complex media for growth, preferably supplemented with blood. More than 40 species of streptococci have been described. Species of streptococci vary in host tropism and virulence properties, and so it is important that they be discussed separately. One of the most important streptococcal pathogens of humans, Streptococcus pyogenes, which causes streptococcal pharyngitis (“strep throat”), does not infect dogs and cats to a significant degree (see Public Health Aspects). However, interspecies transmission has been reported for some pathogenic streptococci such as Streptococcus pneumoniae and Streptococcus equi subsp. zooepidemicus. As is the case in humans, streptococci that infect dogs and cats range from commensals of low virulence through to highly virulent organisms that can cause severe disease manifestations and death. FIGURE 34-1 Histopathology of the serosal surface of the liver of a 12-day-old domestic shorthair kitten that was euthanized after it was found hypothermic and tachypneic. Severe suppurative omphalophlebitis and peritonitis was present. Lesions contained numerous chains of gram-positive cocci. Brown and Benn stain, 1000× oil magnification. FIGURE 34-2 Electron micrograph of Streptococcus pyogenes (A) and Streptococcus canis (B). Proteinaceous cell surface fibrillae are present on S. canis that resemble the M-protein surface fibrils of the human pathogen S. pyogenes. M-protein is a key virulence factor of S. pyogenes. (From DeWinter LM, Low DE, Prescott JF. Virulence of Streptococcus canis from canine streptococcal toxic shock syndrome and necrotizing fasciitis. Vet Microbiol 1999;70:95-110.) Streptococci have been classified based on their hemolytic properties into β-hemolytic streptococci, which cause complete hemolysis when they grow on blood agar; γ-hemolytic or nonhemolytic streptococci, which do not cause hemolysis; and α-hemolytic streptococci, which reduce hemoglobin, causing a greenish discoloration of the agar around bacterial colonies. Streptococci are also classified from A through W based on their surface antigens (Lancefield classification system) and classified based on phenotypic characteristics (such as pyogenic or viridans group streptococci). Major pathogenic streptococci of dogs and cats belong to Lancefield groups B, C, D, or G (Table 34-1). Pyogenic streptococci are β-hemolytic streptococci that belong to Lancefield groups A (S. pyogenes), B (Streptococcus agalactiae), C (which includes Streptococcus dysgalactiae and S. equi subsp. zooepidemicus), and G (which includes Streptococcus canis). Viridans streptococci are often nonhemolytic or α-hemolytic (viridis = Latin for green) and tend to be commensals that have low virulence and rarely invade tissues opportunistically. Many group D streptococci have now been classified as enterococci. Molecular typing schemes have revealed that hemolytic reactions, Lancefield antigens, and phenotypic characteristics do not necessarily predict genetic relatedness among streptococcal strains. Nevertheless, these traditional classification methods are still used by laboratory staff, microbiologists, and clinicians to describe streptococci. Streptococci invade tissues opportunistically when there is a breach in normal host barriers. This leads to a variety of clinical disease manifestations, such as pyoderma, pneumonia, endocarditis, arthritis, osteomyelitis, meningoencephalitis, cellulitis, and urinary tract infections (UTIs). Severe and life-threatening manifestations of streptococcal infection include necrotizing fasciitis (NF) and streptococcal toxic shock syndrome (STSS). Predisposing conditions for streptococcal infections in dogs and cats may include atopic dermatitis, wounds, foreign bodies (such as migrating grass awns), immunosuppressive drug treatment, and young age (especially neonates).3 The most common clinical presentation among fetuses and neonates is streptococcal bacteremia and sepsis, which can result in abortion and death. Streptococcal pneumonia may occur in association with infections with other respiratory pathogens, such as respiratory viruses, or occur secondary to bronchitis, hematogenous spread or aspiration. In one study, streptococci were isolated from sick dogs more frequently in summer months.3 S. canis is the most frequently isolated streptococcus from dogs and cats.3 It is a β-hemolytic, group G (pyogenic) streptococcus that colonizes the skin, genital, and gastrointestinal tracts of healthy dogs and cats. It has also been isolated from other animal species, such as rats, mice, rabbits, mink, and foxes. Infection with S. canis may be associated with neonatal bacteremia, pharyngitis, cervical lymphadenitis, infective endocarditis, UTIs, postoperative incision or wound infections, otitis externa, keratitis, bronchopneumonia, pyometra or metritis, meningoencephalitis, NF, STSS, rhinitis and necrotizing sinusitis, pyothorax, discospondylitis, arthritis, osteomyelitis, mastitis, cholangiohepatitis, and peritonitis. Neonatal infections can occur when organisms are transmitted from the vaginal tract during parturition. The organism can then gain access to the systemic circulation via the umbilical vein. Streptococcal meningitis results from direct extension from the sinuses or middle ear, or from bacteremia. Other embolic complications may accompany bacteremia with group G streptococci. Although opportunistic infections with S. canis occur sporadically, outbreaks of group G streptococcal infection have been reported in group-housed animals, which suggested spread of a virulent strain.4–6 Severe manifestations of S. canis infection, such as STSS and NF, have been increasingly described in dogs and cats in recent years, sometimes in the absence of obvious immunosuppressive underlying conditions or wounds.6–9 Although toxic shock syndrome can also be caused by staphylococci, STSS is defined as any streptococcal infection associated with the sudden onset of shock and organ failure. Mechanisms of shock and organ failure identified in human group A streptococcal infections include elaboration of pyrogenic exotoxins by streptococci, which act as superantigens. Superantigens stimulate T cell responses through their ability to bind to, and cross-link, the MHC class II complex of antigen-presenting cells, and the T cell receptor, which bypasses normal MHC-restricted antigen processing. This leads to sudden and massive cascade of cytokine release, which in turn causes signs of fever, vomiting, and hypotension, together with tissue damage, disseminated intravascular coagulation (DIC), and multiple organ dysfunction.10 Other streptococcal virulence factors also contribute to proinflammatory cytokine release and the development of hypotension. Laboratory abnormalities include thrombocytopenia, azotemia, hypoalbuminemia, and metabolic acidosis. Death can occur within 48 hours after the onset of illness. Specific criteria are used for diagnosis of STSS in human patients, and similar definitions could be used for diagnosis of STSS in dogs and cats (Box 34-1). Because only a few dogs and cats with STSS have been described, predisposing factors have not been clearly identified. Predisposing conditions in humans include diabetes mellitus, alcoholism, surgical procedures, penetrating and nonpenetrating trauma, viral infections such as varicella, and possibly the use of nonsteroidal antiinflammatory drugs. NF is a bacterial infection of the deep subcutaneous tissues and fascia, characterized by extensive necrosis and gangrene of the skin and underlying tissues. NF often begins as a minor wound and progresses rapidly over 24 to 72 hours, and it may be accompanied by STSS (Figure 34-3). The popular term “flesh-eating bacteria” has been used to describe the organisms involved. Streptococcal NF has been described in both dogs and cats. Lesions usually involve a limb and are intensely painful, with localized heat and swelling and accumulation of exudate along fascial planes that requires drainage and debridement. In some dogs, extensive sloughing of necrotic skin occurs.9 Necrotizing myositis, bacteremia, and septic emboli may accompany NF.8 Outbreaks of NF, arthritis, sinusitis, and meningitis caused by S. canis have been reported in cats in shelters5 and breeding colonies.11 Molecular typing (by pulsed-field gel electrophoresis) of isolates from one outbreak revealed that isolates were clonally related, which indicated spread of a virulent strain.12 FIGURE 34-3 A, Two-year-old female spayed domestic shorthair that was euthanized with necrotizing cellulitis, myositis and fasciitis, and peritonitis that developed after an ovariohysterectomy site became infected with Streptococcus canis. B, Histopathology showed severe necrotizing cellulitis, myositis, and cellulitis with intralesional cocci that sometimes formed chains. H&E stain, 1000× oil magnification. (Courtesy of the University of California, Davis, Veterinary Anatomic Pathology Service.) Despite the recognition of severe disease manifestations in some dogs and cats, relatively little is known about virulence factors of S. canis. A protein analogous to M protein, a major virulence factor of S. pyogenes, has been identified in S. canis and was shown to bind plasminogen and degrade thrombi.13 M protein has important antiphagocytic properties. Genes that encode M protein and a streptococcal hemolysin, streptolysin O, have been detected in S. canis isolates from dogs with NF and STSS.14 Genes that encode other virulence factors identified in S. pyogenes, such as pyrogenic exotoxins (Spe genes), streptococcal superantigen (SSA), streptokinase (Ska), and C5a peptidase (Scp, which cleaves the fifth component of complement) have not been detected. S. equi subsp. zooepidemicus is a β-hemolytic, group C streptococcus that is a commensal of the upper respiratory and lower genital tracts of horses, and appears to be an emerging pathogen of dogs and cats. Opportunistic infections such as wound infections, respiratory disease, endometritis, and abortion occur in horses. Infections have also been reported in a variety of other animal species, including cattle, sheep, pigs, monkeys, seals, ruminants, dogs, cats, and humans. Different strains of S. equi subsp. zooepidemicus exist that may vary in pathogenicity and host tropism. Infected horses may have been a source of infection for dogs in some situations,15,16 but in other situations where infected dogs and cats have been identified, a history of horse contact was not present. Several outbreaks of hemorrhagic, fibrinosuppurative, and necrotizing pneumonia associated with S. equi subsp. zooepidemicus infection have been described in group-housed dogs, such as those in kennels and shelter environments, from a variety of geographic locations worldwide (Figure 34-4).17 The pneumonia progresses rapidly in some dogs can be accompanied by signs suggestive of STSS, such as fever and systemic hypovolemia. In some dogs, severe tachypnea and death occurs within 48 hours of the first signs of respiratory disease, and dogs may be found dead without any preceding clinical signs. Other infected dogs show only mild signs of respiratory disease. S. equi subsp. zooepidemicus bacteremia and sepsis has been reported in racing greyhounds.18 Chronic lymphoplasmacytic rhinitis, sometimes with turbinate lysis, has also been described in several dogs in association with S. equi subsp. zooepidemicus infection, which resolved after specific antimicrobial treatment.15,16 Outbreaks of pneumonia, purulent rhinosinusitis, and meningoencephalitis have been described in cats.19,20 S. equi subsp. zooepidemicus meningoencephalitis occurred in a cat as a result of extension of otitis media/interna.21 FIGURE 34-4 Pneumonia in a shelter dog caused by Streptococcus equi subsp. zooepidemicus. The lungs are consolidated and dark red. (Courtesy of Dr. Patricia Pesavento, University of California, Davis.) The pathogenesis of infection with S. equi subsp. zooepidemicus in dogs and cats is unclear. Co-infection with other contagious respiratory pathogens can contribute to the severity of disease. Co-infections with canine distemper virus (CDV), canine adenovirus-2 (CAV-2), canine herpesvirus-1 (CHV-1), canine influenza virus (CIV), Bordetella bronchiseptica, or Mycoplasma cynos have been documented in some, but not all, affected dogs with S. equi subsp. zooepidemicus pneumonia. Experimentally, co-infection of dogs with both CIV and S. equi subsp. zooepidemicus is associated with more severe clinical manifestations of disease than when either organism is inoculated alone.22 Other environmental factors such as overcrowding may also contribute to stress and severe disease manifestations. Superantigen genes have been detected in some isolates of S. equi subsp. zooepidemicus from dogs,23,24 but there has been no correlation between the presence of these genes and the severity of histopathologic lesions. However, expression of proinflammatory cytokines such as TNF-α, Il-6, and Il-8 in the lungs of affected dogs suggests that a potent inflammatory response is involved in the pathogenesis of disease.23 Like S. equi subsp. zooepidemicus, S. dysgalactiae is a β-hemolytic, group C streptococcus. There are two subspecies, S. dysgalactiae subsp. equisimilis and S. dysgalactiae subsp. dysgalactiae. After S. canis and S. equi subsp. zooepidemicus, S. dysgalactiae subsp. equisimilis was the third most common β- or γ-hemolytic streptococcal species isolated from dogs at a veterinary teaching hospital in the United States, and was detected in 13 (3.3%) of 393 dogs with streptococcal infections. Affected dogs had bacteremia and sepsis, dermatitis, or pneumonia.3 S. dysgalactiae subsp. dysgalactiae has been reported in association with systemic neonatal infections and death in a litter of great Dane puppies.25 The porcine streptococcus, Streptococcus suis, has been isolated from cats with meningoencephalitis, pyothorax, or dermatitis.26,27 S. suis was also isolated from the urine of a systemically unwell dog in Canada28 and can be isolated from the tonsils, anus, and skin of healthy dogs and cats.29 Although contact with pigs was not reported in the sick dogs and cats, the dog was fed commercial pig ear treats. S. suis has been classified into serotypes, which have epidemiologic importance. Human infections are usually serotypes 2 and 14. The cases reported in dogs and cats have not belonged to these serotypes. Other streptococcal species isolated from dogs and cats include the human pathogen Streptococcus constellatus (a Streptococcus anginosus group organism), which was isolated from a dog with pyoderma;30 and group B streptococci (S. agalactiae), which have been isolated from dogs and cats with streptococcal disease such as endocarditis and neonatal sepsis. Group B streptococci are important causes of puerperal sepsis and neonatal sepsis and meningitis in humans, as well as mastitis in cattle. Streptococcus bovis group organisms are α- or nonhemolytic and usually possess Lancefield group D antigens. Biotypes that belong to this group, which differ in their biochemical characteristics, have now been reclassified as distinct species, Streptococcus gallolyticus and Streptococcus infantarius.31 S. bovis group organisms are commensals of the gastrointestinal tracts of humans and other animals, especially ruminants. They are important causes of bacteremia and endocarditis in humans, accounting for 11% to 17% of all endocarditis cases. They frequently affect multiple valves and are associated with embolic complications. For unknown reasons, fecal carriage of S. bovis group organisms by humans has been strongly associated with colorectal cancer. This especially seems to be true for S. gallolyticus subsp. gallolyticus.32 Rarely, S. bovis group organisms are isolated from dogs and cats with UTIs. They have also been isolated from the blood of dogs with mitral valve endocarditis, one of which also had sternebral osteomyelitis.33,34 Enterococci are α- or nonhemolytic gram-positive bacteria that usually possess Lancefield group D antigens, are commensals of the gastrointestinal tracts of humans and other animals, and are important nosocomial pathogens. They form pairs or short chains of organisms and cannot morphologically be discriminated from streptococci. Enterococci survive harsh environmental conditions; they grow in medium that contains 6.5% NaCl, can grow at temperatures that range from 10°C to 45°C (50°F to 113°F), and hydrolyze esculin in the presence of 40% bile salts. The genus Enterococcus was separated from Streptococcus in the mid-1980s based on the results of DNA hybridization experiments.35 Enterococci are often resistant to a wide variety of different antimicrobial drug classes, and multiple drug resistant (MDR) enterococcal infections, especially those that are also resistant to vancomycin (vancomycin-resistant enterococci or VRE), are a significant problem in human medicine. Of great clinical importance, resistance among enterococci occurs as a result of both intrinsic and acquired resistance mechanisms. Intrinsic resistance to low levels of most β-lactam antimicrobials occurs because enterococci possess low-affinity penicillin binding proteins (PBPs). Thus penicillins are generally bacteriostatic, not bactericidal, against enterococci. Enterococci also have low-level intrinsic resistance to aminoglycosides, which results from decreased drug uptake. However, uptake of aminoglycosides is enhanced when enterococci are exposed to β-lactams, which explains the synergistic activity of this combination. Enterococci are resistant to trimethoprim-sulfamethoxazole because they utilize exogenously produced folate. Thus, even if the organisms appear sensitive to trimethoprim-sulfamethoxazole in vitro (when exogenous folate is lacking), the drug is not effective in vivo. Many laboratories therefore do not report minimum inhibitory concentrations for this organism-drug combination. Enterococcal resistance to other antimicrobials, such as macrolides and vancomycin, can result from acquired resistance mechanisms. Extensive multidrug resistance has been well documented in enterococci isolated from dogs and cats, but there are only a few reports of enterococci with acquired vancomycin resistance from dogs in the literature. These have been from the United Kingdom, New Zealand, and the United States.36–38 All other isolates examined have been vancomycin susceptible. In contrast, VRE account for about 30% of enterococcal infections in humans from Europe and North America.39 Clinical microbiology laboratories often do not differentiate between Enterococcus species, because definitive identification can require genetic typing. Some laboratories use biochemical methods to identify Enterococcus faecalis and Enterococcus faecium, which are the most prevalent species in humans and which differ in their epidemiology, antimicrobial susceptibility, and virulence properties. E. faecium is more likely to show high-level resistance to penicillins and carbapenems than E. faecalis, and as a result, in humans, the prognosis for serious E. faecium infections may be worse than that for E. faecalis infections.40 However, E. faecalis is more likely to produce biofilms than E. faecium.41 Although most infections in humans have been E. faecalis, a worrisome increase in the prevalence of MDR E. faecium infections has occurred. In particular, ampicillin- and fluoroquinolone-resistant E. faecium strains that belong to hospital adapted strains known as clonal complex 17 (CC17) have emerged. CC17 isolates have been found in dogs, but their virulence gene profile and antimicrobial susceptibility profiles have differed from those of human isolates.42 In healthy dogs and cats, enterococci can be found on the skin and within the oral cavity, nasal cavity, and gastrointestinal tract. In the United States, the most prevalent species in dogs appears to be E. faecalis, which comprised 68% of 155 isolates.43 In the cat, it was Enterococcus hirae, which comprised 52% of 121 isolates.43 Preparations that contain E. faecium strain SF68 have been investigated as probiotics in dogs and cats, with mixed results.44–46 Some studies suggest that supplementation of food with this probiotic can stimulate local and systemic immune function in dogs and cats.47,48 Although less pathogenic than many streptococci, important virulence factors have been identified in enterococci that enable them to invade tissues and cause disease. These include a cytolysin, gelatinase, and a serine protease, as well as several surface proteins (e.g., the Ace protein, which allows the organism to adhere to connective tissue components, such as collagen). Enterococci also possess pili that play an important role in adherence. The ability of many enterococci to form biofilms means they can be very difficult to eradicate, because they resist phagocytosis and antimicrobial drugs.41 As in humans, the two most common species of Enterococcus associated with disease in dogs and cats are E. faecalis and E. faecium.36,49 Others are shown in Box 34-2.
Streptococcal and Enterococcal Infections
Etiology, Epidemiology, and Clinical Features
Streptococcus canis
Streptococcus equi subsp. zooepidemicus
Other Streptococcal Species
Streptococcus bovis Group (Streptococcus gallolyticus and Streptococcus infantarius)
Enterococcus spp
Streptococcal and Enterococcal Infections